Physics 6C
advertisement

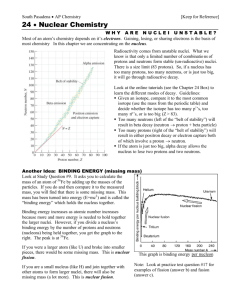
Physics 6C Nuclear Physics and Radioactivity Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Mass Defect (Binding Energy) A stable nuclei is comprised of Z protons and N neutrons, bound together by the strong nuclear force. The fully assembled nucleus is less massive than the individual protons and neutrons, if they are weighed separately. The difference between these masses is called the mass defect. From Einstein’s E=mc2 equation, we can also consider this mass defect to be binding energy. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Mass Defect (Binding Energy) A stable nuclei is comprised of Z protons and N neutrons, bound together by the strong nuclear force. The fully assembled nucleus is less massive than the individual protons and neutrons, if they are weighed separately. The difference between these masses is called the mass defect. From Einstein’s E=mc2 equation, we can also consider this mass defect to be binding energy. As an example, we will calculate the mass defect for oxygen-16, the most common isotope of oxygen. This nucleus has 8 protons and 8 neutrons. We can look up the atomic mass of O-16 in a table (below). This is the mass of a neutral atom of O-16. We will need to account for the 8 electrons in our calculation. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Mass Defect (Binding Energy) Now that we have the mass of the assembled atom (mO16=15.994915u), we need to add up the mass of all the parts. Also listed in a table are the masses we need. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Mass Defect (Binding Energy) Now that we have the mass of the assembled atom (mO16=15.994915u), we need to add up the mass of all the parts. Also listed in a table are the masses we need. We have 8 protons, 8 electrons, and 8 neutrons. As a shortcut we can group the protons and electrons together and use 8 hydrogen atoms instead. Here is the calculation: 8mH 8mn 8(1.007825u) 8(1.008665u) mtotal 16.13192u Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Mass Defect (Binding Energy) Now that we have the mass of the assembled atom (mO16=15.994915u), we need to add up the mass of all the parts. Also listed in a table are the masses we need. We have 8 protons, 8 electrons, and 8 neutrons. As a shortcut we can group the protons and electrons together and use 8 hydrogen atoms instead. Here is the calculation: 8mH 8mn 8(1.007825u) 8(1.008665u) mtotal 16.13192u Now we just subtract to find the defect: m 16.13192u 15.994915u m 0.137005u Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Mass Defect (Binding Energy) Now that we have the mass of the assembled atom (mO16=15.994915u), we need to add up the mass of all the parts. Also listed in a table are the masses we need. We have 8 protons, 8 electrons, and 8 neutrons. As a shortcut we can group the protons and electrons together and use 8 hydrogen atoms instead. Here is the calculation: 8mH 8mn 8(1.007825u) 8(1.008665u) mtotal 16.13192u Now we just subtract to find the defect: m 16.13192u 15.994915u m 0.137005u This value is in atomic mass units. We can also convert to kg or energy, either in Joules or MeV. The conversion factors are also in the book. m (0.137005u)(931.5 MeV ) u m 127.6MeV Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Nuclear Stability Oxygen-16 is here. This chart shows the binding energy per nucleon for all the stable nuclei. The higher the value, the more stable the nucleus will be. Our calculation for oxygen-16 gives 7.98 MeV/nucleon. The most stable nuclei are metals like iron, cobalt and nickel, with values just below 9 MeV/nucleon. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Types of Radioactive Decay Alpha Decay (α) When a nucleus is too large to be stable, it will often emit an alpha-particle, which is really just a helium nucleus (2 protons and two neutrons). The alpha particle carries away some energy, and the resulting nucleus has 2 fewer protons and 2 fewer neutrons (and thus is 2 places lower on the periodic table). Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Types of Radioactive Decay Alpha Decay (α) When a nucleus is too large to be stable, it will often emit an alpha-particle, which is really just a helium nucleus (2 protons and two neutrons). The alpha particle carries away some energy, and the resulting nucleus has 2 fewer protons and 2 fewer neutrons (and thus is 2 places lower on the periodic table). Beta Decay (β) When a nucleus has too many excess neutrons, it may undergo beta-minus decay, whereby a neutron decays into a proton and an electron. The electron is emitted, leaving the nucleus with one more proton than before (and one less neutron). So the result is an atom that is one step higher on the periodic table. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Types of Radioactive Decay Alpha Decay (α) When a nucleus is too large to be stable, it will often emit an alpha-particle, which is really just a helium nucleus (2 protons and two neutrons). The alpha particle carries away some energy, and the resulting nucleus has 2 fewer protons and 2 fewer neutrons (and thus is 2 places lower on the periodic table). Beta Decay (β) When a nucleus has too many excess neutrons, it may undergo beta-minus decay, whereby a neutron decays into a proton and an electron. The electron is emitted, leaving the nucleus with one more proton than before (and one less neutron). So the result is an atom that is one step higher on the periodic table. Gamma Decay (γ) Sometimes another decay process will leave the resulting nucleus in an excited state, when the nucleus drops down a gamma-ray photon is emitted to carry away the excess energy. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Decay Rates For any decay process there will be an associated HALF-LIFE, which describes how long it should take for half of the radioactive nuclei in a sample to undergo the decay. We can keep track of this in several different ways, but it all boils down to the same basic formula. Here is an exponential decay formula: N(t) N0 et This will count the remaining radioactive nuclei in a sample at any given time. You can also use it for the activity of a sample. N0 is the starting amount, and λ is the “decay constant” (related to the half-life). T1 2 ln( 2) The fundamental relationship you need is for the decay rate (this is also called ‘ACTIVITY’: N N t Units for activity are usually decays/second. Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Suppose a radioactive sample has an activity of 100,000 decays per second. When the same sample is measured again 10 days later, the activity has dropped to 80,000 decays per second. What is the half-life of the sample? Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Suppose a radioactive sample has an activity of 100,000 decays per second. When the same sample is measured again 10 days later, the activity has dropped to 80,000 decays per second. What is the half-life of the sample? We can use our basic exponential decay equation and solve for the decay constant: N(t) N0 e t 80,000 100,000 e (10days) Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Suppose a radioactive sample has an activity of 100,000 decays per second. When the same sample is measured again 10 days later, the activity has dropped to 80,000 decays per second. What is the half-life of the sample? We can use our basic exponential decay equation and solve for the decay constant: N(t) N0 e t 80,000 100,000 e (10days) 0.8 e (10days) ln(0.8) (10days) 1 0.0223 day Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Suppose a radioactive sample has an activity of 100,000 decays per second. When the same sample is measured again 10 days later, the activity has dropped to 80,000 decays per second. What is the half-life of the sample? We can use our basic exponential decay equation and solve for the decay constant: N(t) N0 e t 80,000 100,000 e (10days) 0.8 e (10days) ln(0.8) (10days) 1 0.0223 day Now just use our formula for half-life: ln(2) T12 31days 1 0.0223 day Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Carbon-14 is a radioactive isotope with a half-life of 5730 years. It is sometimes used to find the age of archaeological finds. Suppose a bone is dug up and it is determined that 20% of the original amount of 14C remains. How old is the bone? Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Carbon-14 is a radioactive isotope with a half-life of 5730 years. It is sometimes used to find the age of archaeological finds. Suppose a bone is dug up and it is determined that 20% of the original amount of 14C remains. How old is the bone? We can use our basic exponential decay equation and solve for the time. N(t) N0 e t 20% N0 N0 e t 0.2 e t Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Carbon-14 is a radioactive isotope with a half-life of 5730 years. It is sometimes used to find the age of archaeological finds. Suppose a bone is dug up and it is determined that 20% of the original amount of 14C remains. How old is the bone? We can use our basic exponential decay equation and solve for the time. N(t) N0 e t 20% N0 N0 e t 0.2 e t The decay constant is found from the half-life equation. ln(2) ln(2) T12 5730yr 1.21 10 4 1 yr Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Carbon-14 is a radioactive isotope with a half-life of 5730 years. It is sometimes used to find the age of archaeological finds. Suppose a bone is dug up and it is determined that 20% of the original amount of 14C remains. How old is the bone? We can use our basic exponential decay equation and solve for the time. N(t) N0 e t The decay constant is found from the half-life equation. 20% N0 N0 e t 0.2 e t ln(0.2) (1.21 10 4 t 13,300years 1 yr )t ln(2) ln(2) T12 5730yr 1.21 10 4 1 yr Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Radiation Doses When radiation is absorbed by living tissue we need a way to measure the effects. There are two steps to the measurement. 1) How much energy was absorbed (per kg of tissue)? The rad is the more commonly used unit: 1 rad = 0.01 J/kg 2) What type of radiation is it? Different types of radiation affect living tissue in different ways. To account for this we have a multiplicative factor called RBE (relative biological effectiveness). Multiply together to get the “biologically equivalent dose”. Equivalent dose (REM) = RBE X absorbed dose (RAD) An example follows… Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Alpha-particles with an RBE of 13 deliver a 32 mrad whole-body radiation dose to a 69 kg patient. a) What dosage, in rem, does the patient receive? b) How much energy is absorbed by the patient? Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Alpha-particles with an RBE of 13 deliver a 32 mrad whole-body radiation dose to a 69 kg patient. a) What dosage, in rem, does the patient receive? b) How much energy is absorbed by the patient? For part a) we multiply to get the equivalent dose in rems: dose (13) (32 10 3rad) 0.416rem Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB Example: Alpha-particles with an RBE of 13 deliver a 32 mrad whole-body radiation dose to a 69 kg patient. a) What dosage, in rem, does the patient receive? b) How much energy is absorbed by the patient? For part a) we multiply to get the equivalent dose in rems: dose (13) (32 10 3rad) 0.416rem For part b) just use the definition of rad: J 1rad 0.01 kg J ) 3.2 10 4 32mrad (32 10 3 )(0.01 kg Eabsorbed (69kg)(3.2 10 4 J) kg J kg 0.022 J Prepared by Vince Zaccone For Campus Learning Assistance Services at UCSB