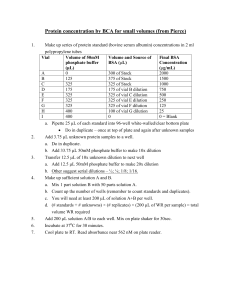
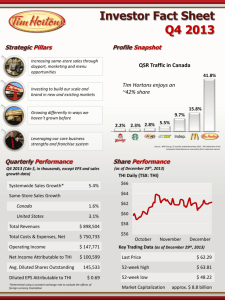
Bacteria Predominate
advertisement

Arabidopsis Experiments: I. Forward Genetic Screen (Ethylene Insensitive Mutants): • requires thinking. II. Reverse Genetic Screen / PCR Genotyping (H+- ATPase Mutants): • requires scoring F2 and thinking. What Next? …experiment I dominant Thought Experiments… Backcross to wild-type, what might the F1 and F2 tell us? Complementation tests? Given etr1, etr2, ers1, ers2, ein4, ctr1, ein2, ein3, eil1 and erf1 homozygous plants, and wt plants; devise a plan to describe the genetic nature of the 12 long hypocotyl mutants you found. recessive What Next? …experiment II L t T homozygote wt 5’ 5’ 3’ 3’ L t T 5’ 3’ 5’ 3’ heterozygote homozygote mutant 5’ 3’ 5’ 3’ L t T Genetic Analysis F2 Segregation (Friday) …what next? T t T TT Tt t Tt tt 1:2: 1 Not Lethal T t T TT Tt t Tt tt 1 wt : 2 het Lethal How would you confirm / extend F2 results? T t T TT Tt t Tt tt 1 wt : 1 het Gametophyte Lethal Genetic Selection ...the process that establishes conditions in which only the desired genotype will grow. Selective Media: what might this be? Genetic Screen • A system that allows the identification of rare mutations in large scale searches, – unlike a selection, undesired genotypes are present, the screen provides a way of “screening” them out. The (Awesome) Power of Bacterial Genetics ... is the potential for studying rare events. Liquid Cultures, • 109cells/microliter, Colonies on Agar, • 107+ cells/colony Counting Bacteria 10-3 10-4 10-5 (Serial) Dilution is the Solution Extra Credit: On another piece of paper, answer the dilution problems on the last page of your handout (2 pts), due Thursday, 13th. Bacteria Phenotypes • colony “morphology”, – large, small, shiny, dull, round or irregular, – resistance to bactericidal agents, – vital dyes, • auxitrophs, – unable to synthesize or use raw materials from the growth media. Prototroph …a cell that is capable of growing on a defined, minimal media, – can synthesize all essential organic compounds, – usually considered the ‘wild-type’ strain. Auxotrophs …a cell that requires a substance for growth that can be synthesized by a wild-type cell, hisleuargbio- ...can’t synthesize histidine (his+ = wt) ...can’t synthesize leucine (leu+ = wt) ...can’t synthesize arginine (his+ = wt) ...can’t synthesize biotin (bio+ = wt) Bacterial Nomenclature • genes not specifically referred to are considered wildtype, – Strain A: met bio (require methionine and biotin) – Strain B: thr leu thi • bacteriacide resistance is a gain of function, – Strain C: strA (can grow in the presence of strptomycin). Conjugation ...temporary fusion of two single-celled organisms for the transfer of genetic material, …the transfer of genetic material is unidirectional. + - F Cells F Cells (F for Fertility) (F for Fertility) … F+ cells donate genetic material. … F- cells receive genetic material, …there is no reciprocal transfer. F+ F Pilus F - …a filamentlike projection from the surface of a bacterium. F Factor …a plasmid whose presence confers F+, or donor ability. - F Pilus Attaches to F Cell F Factor Replicates During Binary Fission Properties of the F Factor • Can replicate its own DNA, • Carries genes required for the synthesis of pili, • F+ and F- cells can conjugate, – the F factor is copied to the F- cell, resulting in two F+ cells, • F+ cells do not conjugate with F+ cells, • F Factor sometimes integrates into the bacterial chromosome creating Hfr cells. Hfr Cells F factor ...F factor integration site, ...host (bacteria chromosome) integration site. Bacterial Chromosome Inserted F plasmid ’ F Cells • an F factor from an Hfr cell excises out of the bacterial genome and returns to plasmid form, • often carries one or more bacterial genes along, • F’cells behave like an F+ cells, – merizygote: partially diploid for genes copied on the F’plasmid, • F’plasmids can be easily constructed using molecular biology techniques (i.e.vectors). Transfer of lac+pro+ from a F' to an F- strain. • • • Strain CSH23 CSH50 Sex F’ lac+ proA+ proB+ F- Genotype D(lacpro) supE spc thi ara D(lacpro) strA thi strA: confers resistance to streptomycin spc: confers resistance to spectinomycin D indicates a deletion of the genes in parentheses lac: cannot utilize lactose as a carbon source pro: indicates a requirement for proline thi; indicates a requirement for thiamine supE: suppresses nonsense mutations ara: cannot utilize arabinose as a carbon source. • Strain F’ genotype Chromosome Genotype CSH23 F’lac+ proA+ proB+ D(lacpro)supE spc thi x ara D(lacpro)strA thi CSH 50: Conjugation Recombinant Strain: F’lac+ proA+ proB+ ara D(lacpro)strA thi Procedure I: • Day 0: Overnight cultures of the CSH23 and CSH50 will be set up in L broth (a rich medium). • Day 1: These cultures will be diluted and grown at 37o until the donor culture is 2-3 X 108 cell/ml. What is the quickest way to quickly determine #cells per ml? (This will be done for you.) Prepare a mating mixture by mixing 1.0 ml of each culture together in a small flask. Rotate at 30 rpms in a 37o shaking incubator for 60 minutes. At the end of the incubation… Do serial dilutions: • Fill 6 tubes with 4.5 ml of sterile saline. Transfer 0.5 ml of the undiluted mating culeture to one of the tubes. This is a 10-1 dilution. • Next make serial dilutions of 10-2, 10-3, 10-4, 10-5 & 10-6. Always change pipets and mix well between dilutions. Procedure II: • • • • Plate: 0.1 ml of a 10-2, 10-3 and 10-4 dilution onto minimal + glucose + streptomycin + thiamine. Plate: 0.1 ml of a 10-5 and 10-6 dilution onto a MacConkey + streptomycin plates. [A MacConkey plate is considered a rich media. It has lactose as well as other carbon sources. The phenol red dye is present to differentiate lac+ colonies (red) from lac- colonies (white).] • Controls: Plate: 0.1 ml of a 10-1 dilution of donor (CSH23) cells on minimal + glucose + strep + thiamine plates. Repeat for the recipient (CSH50) cells. Plate: 0.1 ml of a 10-5 dilution of the recipient on a MacConkey + strep plate. Plate: 0.1 ml of a 10-1 dilution of donor on a MacConkey + strep plate. • Place all plates at 37o overnight. • Day 2: Remove the plates from the incubator the next day and count the number of white-clear colonies on the MacConkey plates (optional but easier). Store plates at 4oC. NOTE: MacConkey color reactions fade after several days or rapidly in the cold, so plates need to be scored soon after incubation. • What’s Growing? …mate in rich, transfer to… • Plate: minimal + glucose + streptomycin + thiamine: – CSH23 yes / no – CSH50 yes / no – exconjugate yes / no • Plate: MacConkey (rich) + streptomycin plates: – CSH23 yes / no – CSH50 yes / no – exconjugate yes / no CSH23 F’lac+ proA+ proB+ D(lacpro)supE spc thi x ara D(lacpro)strA thi CSH 50: F’lac+ proA+ proB+ ara D(lacpro)strA thi Extra Credit • On another piece of paper, answer the dilution problems on the last page of your handout (2 pts), due with your abstract on Weds.