High Frequency of Recombination (Hfr)
advertisement

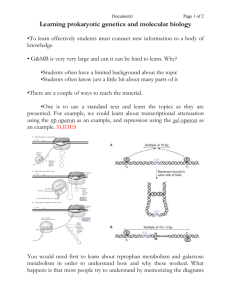
This Week • Score Conjugation Plates, • Start High Frequency of Recombination (HFR) experiment, • Continue Nasonia experiment. High Frequency of Recombination (Hfr) ...bacteria exhibiting a high frequency of recombination, – an alteration DNA sequence such that the genotype of subsequent individuals differs from the parent, …specifically, strains with a chromosome integrated F factor that is able to mobilize and transfer part of the chromosome to the F- cell. Hfr Cells F factor ...F factor integration site, ...host (bacteria chromosome) integration site. Bacterial Chromosome Inserted F plasmid - F Pilus Attaches to F Cell Hfr DNA is Cut F factor and Chromosomal DNA are Transferred Recombination Requires Crossing over Double Crossover DNA not Incorporated into Chromosome are Digested F factor inserts in different regions of the bacterial chromosome, Also inserts in different orientations. Origin of Replication Hfr strain H 1 2 3 Order of transfer thr azi ton lac pur gal his gly thi thr thi gly his gal pur lac ton azi lac pur gal his gly thi thr azi ton gal pur lac ton azi thr thi gly his Indicates direction of transfer. F factor Aa A aA Hfr F- Hfr DNA that is not incorporated in the F- strand, and DNA that has crossed out of the F- strand is digested. F factor A transfers first. A A Hfr F- A transfers last. A Hfr A F- Leading Gene: the first gene transferred is determined empirically. Hfr strain H 1 2 3 Order of transfer thr azi ton lac pur gal his gly thi thr thi gly his gal pur lac ton azi lac pur gal his gly thi thr azi ton gal pur lac ton azi thr thi gly his E. coli Map • 0 minutes is at the threonine, • 100 minutes is required to transfer complete genome, Assignments Bacteria II Lab Report (last page ho), with maps, is due 5/28/10, pp. 3 assignment (Bacteria II) due 5/21/10 Spontaneous Mutations Mutation: an inheritable change in the DNA sequence of a chromosome. DNA replication in E. coli occurs with an error every ~ 109 bases. • - The E. coli genome is 4.6 x 106 bases. – an error occurs once per ~ 200 replications. • - If a single colony has 107 bacteria, • 500 cells carry a mutation, – or, one mutation every ~ 100 bases (across a colony), – or, at least a mutation in about every gene (in a colony). Induced Mutations • Ethylmethane sulfonate (EMS), – EMS adds an ethyl group to G and T residues, allowing the modified base to base-pair inappropriately. Question: how much higher is the rate of mutation after mutagenic treatment? Mutagenesis • Part I: Viable cell counts • Untreated culture Do a serial dilution of the untreated wildtype E. coli culture: Fill 7 tubes with 4.5 ml of sterile saline. Transfer 0.5 ml of the undiluted culture to one of the tubes. This is a 10-1 dilution. Next make serial dilutions of 10-2, 10-3, 10-4, 10-5, 10-6 and 10-7. Always change pipets and mix well between dilutions. • Plate 0.1 ml of the 10-6 onto an L plate. • Repeat for the 10-7 dilution. • Place the plates at 37oC overnight. • EMS-treated culture • You will be given an EMS treated culture. Do a viable cell count on this culture using the same dilutions as described above. Rifampin, Rifamycin, Rifampicin, Rifabutin (bactericidal) • Rifampin (RIF) is a first-line antituberculosis drug, – resistance to RIF, in the majority of cases, has been associated with mutations within an 81-bp RIF resistance-determining region (RRDR) of the rpoB gene, which encodes the ß subunit of the RNA polymerase (1,342 bp). – RIF acts by binding to the ß subunit of the RNA polymerase, thus interfering with transcription and RNA elongation. • Part II: Selection for rifR mutants: • RifR mutants: Rifampcin is a potent inhibitor of E. coli RNA polymerase. Mutants of E. coli that are resistant to this antibiotic have been isolated and shown to have an altered RNA polymerase. • Untreated culture To select for spontaneous rifampicinresistant mutations: Spread 0.2 ml of undiluted culture on an L plate that contains rifampicin (100 g/ml). Set up a total of 2 such plates. Place the plates at 37oC overnight. • EMS-treated culture To select for rifampicin-resistant cells: • Spread 0.1 ml of each of the following dilutions on an L plate that contains rifampicin (100 g/ml): undiluted, 10-1, 10-2, 10-3. • Place the plates at 37oC overnight. Regulation of prokaryotic transcription 1. Single-celled organisms with short doubling times must respond extremely rapidly to their environment. 2. Half-life of most mRNAs is short (on the order of a few minutes). 3. Coupled transcription and translation occur in a single cellular compartment. Therefore, transcriptional initiation is usually the major control point. Most prokaryotic genes are regulated in units called operons (Jacob and Monod, 1960) Operon: a coordinated unit of gene expression consisting of one or more related genes and the operator and promoter sequences that regulate their transcription. The mRNAs thus produced are “polycistronic’—multiple genes on a single transcript. The metabolism of lactose in E. coli & the lactose operon …very short course. LacZ: -galactosidase; Y: galactoside permease; A: transacetylase (not required for lactose catabolism), P: promoter; O: operator, LacI: repressor; PI and LacI are not part of the operon. IPTG: nonmetabolizable artificial inducer (can’t be cleaved) Negative regulation of the lac operon ~6,000 bp • Part III: Screen for lac- + lac- mutants • lac-mutants: Wild-type lac+ colonies appear dark red on MacConkey indicator plates. Mutant colonies that are not capable of utilizing lactose as an energy source will appear as white colonies on MacConkey plates. • • • • • Untreated culture Spread 0.1 ml of the 10-5 dilution on a MacConkey plate. Also, spread 0.1 ml of the 10-6 dilution on a MacConkey plate. Set up a total of 3 plates of each dilution. Place the plates at 37oC overnight. • Remove the plates from the incubator the next day. Score immediately for white colonies. Streak out each candidate lac- mutant on a MacConkey plate to confirm the lac- phenotype and to isolate single colonies. Place at 37oC overnight. Remove the next day and store at 4oC. • EMS-treated culture • Follow the instructions for the untreated culture. • PART IV: SELECTION FOR LAC- LAC+ REVERTANTS • Grow up an overnight culture of your lac- strain in minimal medium plus glucose. • Next day do a viable cell count on the culture as described previously and set up an experiment to identify revertants – how would you select for revertants? – how would you screen for revertants? • REPORT DUE: 6/2/10