Protein (BCA) assay on microtiter plate
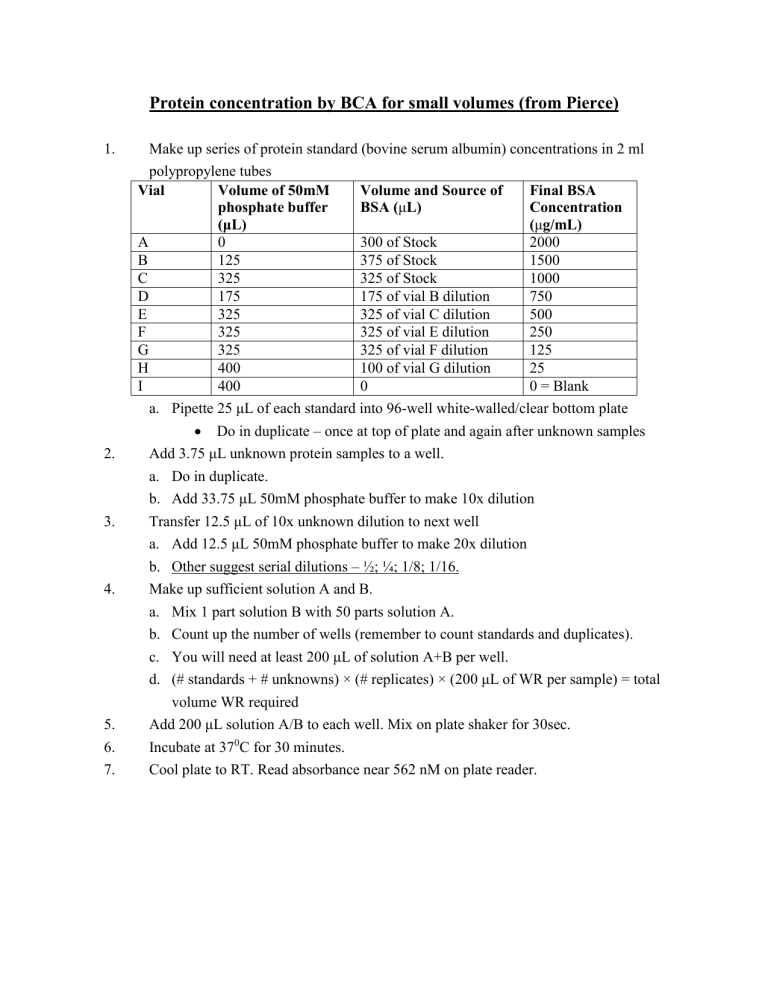
Protein concentration by BCA for small volumes (from Pierce)
1.
Make up series of protein standard (bovine serum albumin) concentrations in 2 ml polypropylene tubes
Vial
A
B
C
D
E
F
G
H
I
Volume of 50mM phosphate buffer
(μL)
0
125
325
175
325
325
325
400
400
Volume and Source of
BSA ( μ L)
300 of Stock
375 of Stock
325 of Stock
175 of vial B dilution
325 of vial C dilution
325 of vial E dilution
325 of vial F dilution
100 of vial G dilution
0
Final BSA
Concentration
(
μ g/mL)
2000
1500
1000
750
500
250
125
25
0 = Blank a.
Pipette 25 μL of each standard into 96-well white-walled/clear bottom plate
Do in duplicate – once at top of plate and again after unknown samples
2.
Add 3.75 μL unknown protein samples to a well. a.
Do in duplicate. b.
Add 33.75 μL 50mM phosphate buffer to make 10x dilution
3.
Transfer 12.5 μL of 10x unknown dilution to next well a.
Add 12.5 μL 50mM phosphate buffer to make 20x dilution b.
Other suggest serial dilutions – ½; ¼; 1/8; 1/16.
4.
Make up sufficient solution A and B. a.
Mix 1 part solution B with 50 parts solution A. b.
Count up the number of wells (remember to count standards and duplicates). c.
You will need at least 200 μL of solution A+B per well. d.
(# standards + # unknowns) × (# replicates) × (200 μL of WR per sample) = total volume WR required
5.
Add 200 μL solution A/B to each well. Mix on plate shaker for 30sec.
6.
Incubate at 37
0
C for 30 minutes.
7.
Cool plate to RT. Read absorbance near 562 nM on plate reader.











