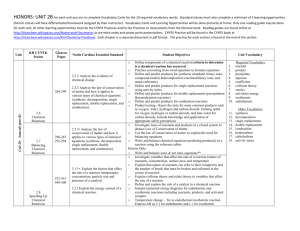
backstroker1994.cnemicalreactionprimer
advertisement

Written, Researched, and Compiled ByStorm Roberts Chemical Reaction Basics: Chemical Reaction Basics What is a chemical reaction? A chemical reaction is the joining of 2 or more reactants (atoms) in order to create a product (compound). Chemical reactions can also be the separation of a product in order to produce the two reactants you started with. Chemical Reaction Basics What parts of the Atom are effected by the chemical reaction? The part of the atom that is effected is the electron. These particles either form bonds with electrons from the other atoms or break their bonds with other atoms depending on the type of chemical reaction. Chemical Reaction Basics How does the law of mass relate to chemical reactions? This law states that matter can never be destroyed. This relates because it shows that, during the chemical reaction process, no atom disappears (or stops existing) it just joins with another atom. These atoms still have the ability to be separated again in order to prove that no matter was lost. Chemical Reaction Basics Signs of a Chemical Reaction!!: > The production of a precipitate (a solid formed during the mixing of two liquids) > A change in color Chemical Reaction Basics Signs of a Chemical Reaction (cnt.) > A formation of a gas (aka fizzing, boiling, etc) > An increase or decrease in temperature that has noting to do with outside factors. Energy & Chemical Reactions The role of energy in Chemical reactions is displayed by the way energy enables the reaction. Energy is what gets the two reactants to a state where the electrons can interact and start the chemical reaction process. Energy is like the fuel needed to burn in order to cause a chemical reaction. The two main types of energy used are potential and kinetic energy. Energy & Chemical Reactions: Energy & Chemical Reactions Potential Energy > Potential energy is energy that is “stored” in the atoms. This energy has the potential to be released when said atom undergoes a chemical reaction with another atom. Kinetic Energy > Kinetic energy is energy that is “the energy of motion”. Kinetic energy is energy something has when it is motion. When that said something makes contact with something else, some of this energy is transferred. Kinetic energy is the energy that can set an chemical reaction off in the first place. Energy & Chemical Reactions But what do the two types of energies have to do with chemical reactions? Well, kinetic energy is the force that starts the reaction. When the two atoms come together the kinetic energy is shared between the two, and then the potential energy that is stored is set off, and keeps the reaction going. An example of this is a fire and a candle. The fire sets off the candle and the fire on the wick continues to make the candle burn. Energy & Chemical Reactions Exothermic and Endothermic Reactions Exothermic Reactions An Exothermic reaction is a reaction that puts out energy. This energy can be displayed as heat or smoke. An example of this is fire. Endothermic Reactions An endothermic reaction is a reaction in which energy is absorbed. This can be displayed by a drop in temperature. Energy & Chemical Reactions Examples of an Endothermic Reaction Examples of an Exothermic Reaction > melting ice cubes > melting solid salts > evaporating water >mixing concentrated acid with water >oxidation of metals These can be classified as endothermic reactions. More scientific examples consist of dissolving ammonia chloride into water or mixing water and ammonium nitrate. Exothermic Reactions are typically ones that give off heat. Any reactions that result in boiling liquids or a burning flame can be classified as Exothermic reactions. Types of Chemical Reactions: Types of Chemical Reactions There are several types of chemical reactions including: > Synthesis > Decomposition > Combustion > Single Replacement > Double Replacement KOH + H2SO4 ---> K2SO4 + H2O FeS + HCl ---> FeCl2 + H2S NaCl + H2SO4 ---> Na2SO4 + HCl AgNO3 + NaCl ---> AgCl + NaNO3 Types of Chemical Reactions Synthesis: In a synthesis reaction, 2 simple substances combine to form a more complex substance. Two or more reactants making one product is another way to identify a synthesis reaction. An example of this is the combination of a simple hydrogen atom and a simple oxygen makes the complex substance of WATER! (water) (cartoon of synthesis) Types of Chemical Reactions Decomposition: During decomposition, a complex substance breaks down into simpler parts. Decomposition is the opposite type of reaction then synthesis. Decomposition is basically where they take a compound and break it down into the original atoms. ( breaking down of water into hydrogen and oxygen) Types of Chemical Reactions Combustion: A reaction that always involves the molecule O2. Combustion is the reaction that is always equated with burning… in fact, nothing can burn without combustion. Combustion is always a type of exothermic reaction. An example of combustion is the burning of wood. (wood burning illustrated example) (formula for the combustion of rubbing alcohol) Types of Chemical Reactions Single Replacement: Single replacement is when there are reactants in which one is a compound and one is a plain atom. When the two reactants react, one element of the compound attaches to the single atom and the left over atom is alone. In the beginning there are two reactants and in the end there are two products. (Cartoon example of single Replacement) (formula for the single replacement that zinc forms with hydrochloric acid) Types of Chemical Reactions Double Replacement: Double replacement is when the parts of two atoms switch “partners” with each other to form two new compounds. Since there are 2 parts 2 each compound, it would be as if compound AB combined with compound CD to make compound AD and CB. (this is an example of how double replacement would look if it were to happen between silver nitrate and sodium chloride. The two combined to make silver chloride and sodium nitrate) (This is a cartoon demonstration) Examples, Examples, Examples! Examples, Examples, Examples! An example of an endothermic reaction would be the breaking of a cold pack, as displayed in class. This happens because the liquid and solid particles interacted with each other and had a reaction in which the temperature of the new product dropped from the beginning temperature of the two reactants as individuals. Examples, Examples, Examples! An example of an exothermic reaction would be how aluminum reacts with a mixture of water and CuCl2. The aluminum begins to almost burn and rust, it seems and raises the temperature up. In our experiment, the harmless mixture went from a harmless room temperature to a scalding 110°F, which is a large difference of 30°. Examples, Examples, Examples! An example of a synthesis reaction would be the reaction between 4 hydrogen atoms and 2 oxygen atoms. In the end it went from the chemical formula of 2H2 + O2 2H2O2 . This is an example of synthesis because in the end the atoms joined together into a compound. Examples, Examples, Examples! An example of decomposition is shown in the experiment where you heat up sodium bicarbonate (2NaHCO3). You see when you heat this powder up, the different reactants that are in the compound break up into Na2CO3+H2O+CO2. In the end, this is demonstrated by the fact that the water (H2O) is accumulated on the edge of the test tube, and the Carbon Dioxide (CO2) puts out the flame used in the testing. This shows that decomposition is the reaction that occurs. Examples, Examples, Examples! An Example of combustion is the lighting of a magnesium strip on fire. You see, the strip bursts into flames that have intense heat and a bright light. This is combustion because, any reaction putting off fire, is combustion. And clearly, magnesium puts off a flame. Examples, Examples, Examples! An examples of single replacement is an examples we have looked at already. This is the of how aluminum mixes with a CuCl2 solution. You see in the reaction goes like this: 3CuCl2 + 2Al 2AlCl3 + 3Cu. This is causes the aluminum to take on an odd, rusted color and the acid to turn to a greenish color from its original blue. Examples, Examples, Examples! Finally, an examples of the last type of chemical reactions, double replacement, is the mixing of Sodium Sulfate (Na2SO4) and Barium Chloride (BaCl2 to create Barium Sulfate (BaSO4) and Sodium Chloride (2NaCl). This is an example of double replacement because in the reaction each reactant switched the second half A Conclusion (finally!!!) Ok, so, there are so many different types of factors affecting reactions, From energy to quantities, everything can change everything else. There are different types of reactions and also different out comes. Just remember there is always a way to identify the reactions and the changes. Sources (pictures) Fireworks (slide 3) http://disneybear.com/wpcontent/uploads/2006/07/FIREWORKS_2_L%5B1%5D.JPG How to identify a chemical reaction (slides 6-7 first 3 pictures) http://www.harpercollege.edu/tmps/chm/100/dgodambe/thedisk/chemrxn/signs4.htm Heat (slide 7 bottom picture) http://images.google.com/images?gbv=2&hl=en&q=heat Explosion (slide 8) http://upload.wikimedia.org/wikipedia/commons/c/c7/Explosions.jpg Billiards (slide 10) http://66.116.149.136/images/billiards.JPG Ice Cube (slide 12)http://images.google.com/images?gbv=2&hl=en&q=ice+cubes Campfire (slide 12) http://www.dracutforum.net/wpcontent/uploads/2007/08/campfire.jpg Pictures and graphs for slides 16, 17, 19, 20 http://www.usoe.k12.ut.us/curr/science/sciber00/8th/matter/sciber/chemtype.htm Combustion picture and graph(slide 18): http://www.iun.edu/~cpanhd/C101webnotes/chemical%20reactions/combustion.html Picture of cold pack (slide 22) : http://static.howstuffworks.com/gif/cold-pack.jpg Sources (pictures cont.) Picture of thermometer(slide 23): http://images.google.com/images?gbv=2&hl=en&q=thermometer Picture of baking soda (slide 24): http://images.google.com/images?hl=en&q=baking+soda&gbv=2 Picture of test tube (slide 25): http://www.culturemediasupplies.com/TT9800C%20Test%20tube%20small2.jpg Picture of magnesium(slide 26): http://www.culturemediasupplies.com/TT9800C%20Test%20tube%20small2.jpg Picture of aluminum ball (slide 27):http://myscruffer.com/images/FoilBall.jpg Sources (sites) What happens during a chemical reaction: http://wiki.answers.com/Q/What_happens_when_a_chemical_reaction_occurs Definition of reactant: http://chemistry.about.com/library/glossary/bldef7550.htm Definition of product: http://chemistry.about.com/library/glossary/bldef7050.htm Law of Conservation of mass: http://dbhs.wvusd.k12.ca.us/webdocs/Thermochem/LawCons-Mass-Energy.html How to tell there's been a chemical reaction: http://dbhs.wvusd.k12.ca.us/webdocs/Thermochem/Law-Cons-Mass-Energy.html Types of energy: http://www.fordhamprep.com/gcurran/sho/sho/lessons/lesson16.htm Endothermic reactions: http://chemistry.about.com/od/lecturenotesl3/a/endorxns.htm Exothermic reactions: http://chemistry.about.com/od/lecturenotesl3/a/endorxns.htm Definitions of synthesis, decomposition, double replacement, and single replacement: http://www.usoe.k12.ut.us/curr/science/sciber00/8th/matter/sciber/chemtype.htm Definition of Combustion: http://www.iun.edu/~cpanhd/C101webnotes/chemical%20reactions/combustion.html Sources (Others) All examples in the example section were provided by the experiments of one Mr. Wildeboer, from his 9th grade honors integrated science class. Documentation of these examples are provided in the form of a worksheet on the types of reactions. Thank you, Mr. W. for making this information accessible.