Sample Calculations distillation
advertisement

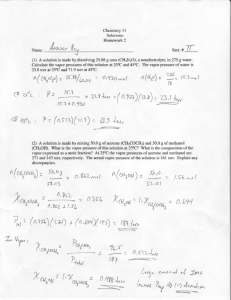
Sample Calculations For concentration of Methanol Set up a Calibration curve and get the second order polynomial that fits: y = -5E-06x2 + 0.0005x + 1.3289 a = -5E-06 b = 0.0005 c = 1.3289 c* = c-y where y is the n value and x is the concentration of Methanol in wt%. 𝑐𝑜𝑛𝑐 𝑜𝑓 𝑀𝑒𝑡ℎ𝑎𝑛𝑜𝑙 = 𝑥 = −𝑏 ± √𝑏 2 − 4𝑎𝑐 ∗ 2𝑎 For n = 1.337 the two roots = c* = 1.3289 – 1.337 = -0.0081 −0.0005 ± √−0.00052 − 4 × (−5𝐸 − 06) × (−0.0081) = 79.66 𝑎𝑛𝑑 20.34 2 × (−5𝐸 − 06) Since it’s the concentration of the first liquid stage a concentration of 20.34 would make more sense because the first stage has a lower Methanol concentration than stages above. Now we need the mole percent from the mass percent. We can set a basis of 1g of Methanol+Water solution and get the mole percent from that. Total Mass of Methanol : 𝑀𝑒𝑂𝐻𝑤𝑡 = 𝑤𝑡% × 𝑡𝑜𝑡𝑎𝑙 𝑤𝑡. 100 .2034 x 1g = .2034 g Methanol Mole Percent of Methanol: 𝑀𝑒𝑂𝐻𝑚𝑜𝑙% 𝑀𝑒𝑂𝐻𝑤𝑡 𝑔 ⁄𝑀𝑊 𝑀𝑒𝑂𝐻 𝑔/𝑚𝑜𝑙 = 100 × 𝑀𝑒𝑂𝐻𝑤𝑡 𝑔 (𝑡𝑜𝑡𝑎𝑙 𝑚𝑎𝑠𝑠 − 𝑀𝑒𝑂𝐻𝑤𝑡 )𝑔 + ⁄𝑀𝑊 ⁄𝑀𝑊 𝑔/𝑚𝑜𝑙 𝑀𝑒𝑂𝐻 𝑔/𝑚𝑜𝑙 𝐻2 0 𝑀𝑒𝑂𝐻𝑚𝑜𝑙% = 100 × .2034𝑔 ⁄32 𝑔/𝑚𝑜𝑙 (1−.2034)𝑔 .2034𝑔 ⁄18𝑔/𝑚𝑜𝑙 ⁄32 𝑔/𝑚𝑜𝑙 + = = 12.56 mol% MeOH To get Murphree Efficiencies: Note: The vapor and liquid stage efficiencies are obtained in the same way by replacing y with x and x with y in the following equation 𝜂 = 𝐸𝑀𝑉 = 𝑦𝑛 − 𝑦𝑛+1 𝑦𝑛∗ − 𝑦𝑛+1 yn = vapor mole fraction at stage n yn+1 = vapor mole fraction at stage n+1 yn* = vapor mole fraction in equilibrium with xn For stage 1, vapor Efficiency: yn = 0.28 xn = 12.56 yn+1 = 0.44 yn* = 0.45 𝜂 = 𝐸𝑀𝑉 = 0.28 − 0.44 = −16 0.45 − 0.44 Operating Lines: Although the column was at total reflux, the distillate concentration could be estimated from stage 6 concentrations (last stage). The equation for the bottom operating line could not be determined because the bottoms concentration was not acquired. Equations for the feed and top operating lines were obtained: Feed line since it is a saturated liquid, it is just a vertical line at x = 0.1 since the feed concentration was 10% Operating line : 𝑦= 𝑅 1 𝑥+ 𝑥 𝑅+1 𝑅+1 𝐷 R = Reflux Ratio y = vapor concentration x = liquid concentration xd = Distillate concentration For this experiment the distillate concentration chosen was 0.9144, this was the concentration of the liquid stage 6. The vapor concentration would have been more ideal but since our data did not give us reasonable data that real vapor concentration could not be used. A reflux value of 1.3379 was used Equation for this experiment: 𝑦= 1.3379 1 𝑥+ . 9144 = .5728𝑥 + .39112 1.3379 + 1 1.3379 + 1