seawaterprop
advertisement
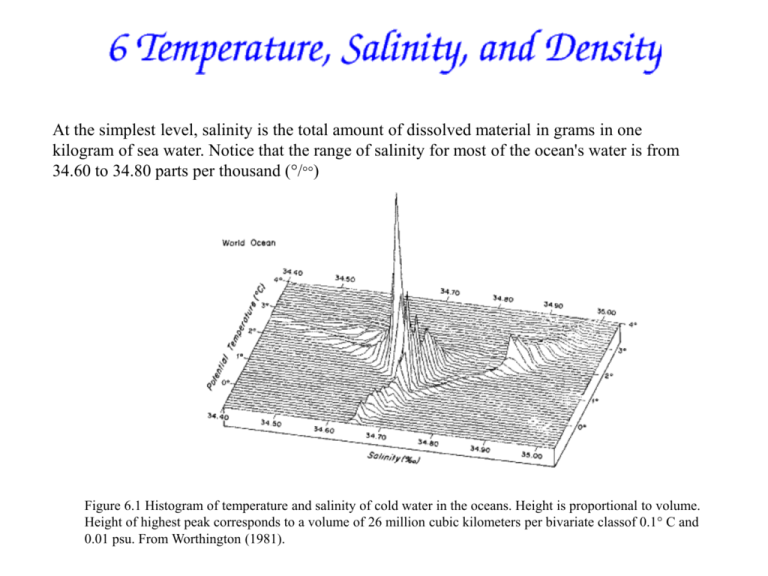
At the simplest level, salinity is the total amount of dissolved material in grams in one kilogram of sea water. Notice that the range of salinity for most of the ocean's water is from 34.60 to 34.80 parts per thousand (°/°°) Figure 6.1 Histogram of temperature and salinity of cold water in the oceans. Height is proportional to volume. Height of highest peak corresponds to a volume of 26 million cubic kilometers per bivariate classof 0.1° C and 0.01 psu. From Worthington (1981). 1. Density is defined as the mass of water per unit volume -use rho (r)as the symbol for density -density units are grams per cubic centimeter (gms / cm3), kilograms per liter (kg/ L) or kilograms per cubic meter (kg/m3) 2. Useful Conversions: 1 cubic meter (m3 ) = 1000 liters (L) 1 L = 1000 cubic centimeters (cm3 ) thus 1 m3 = 1,000,000 cm3 = 106 cm3 3. Water density -density of freshwater at 4°C is 1.0000 gms/cm3 or 1.000 kg/liter or 1000 kg/m3 4. Density range in ocean is from about 1.020 to 1.070 gms/cm3 -the changes in density are caused mainly by variations in pressure, salinity and temperature -colder water more dense -saltier water more dense -higher pressure causes density increase -pressure increases with depth due to the mass of water above 5. Density (r) variations in the ocean are in the parts per thousand range -that is, between 1.020 and 1.070 gm/cm3 the density changes by 50 parts per thousand -(multiply 1.020 and 1.070 by 1000 and subtract one from the other) -we also use s (Sigma) to denote density, where s = (r - 1)*1000 -sigma is useful to express the small changes in density (parts per thousand) that we observe in the ocean -thus if density of water is 1.025 gms/cm3, r = 1.025 gms/cm3 and s = 25 -remember that to convert a s value to density (r) then divide by 1000 and add 1, -e.g. = 28 means r = 1.028 gms/cm3 or 1028 kg/m3 How much does the seawater density increase upon cooling from 20° to 0°C? -seawater density increases from 1.0240 gm/cm3 (1024.0 kg/m3) at 20°C to 1.0273 gm/cm3 (1027.3 kg/m3) at 0°C (at a constant salinity = 34) -thus s increases from 24.0 to 27.3 1 1 7 The potential temperature decrease with depth is greatest in top 1000m (Fig. 6) -the depth region where temp decrease is greatest with depth is called the thermocline -the rate of change of temperature with depth is called the temperature gradient -a gradient expresses the rate of change of one variable relative to another variable, -i.e., in this case the depth gradient in Potential Temperatrue = ∆q/∆Z -the steepness of the depth gradient in temperature depends on location -it is greatest in the warm tropical ocean and least in the cold polar ocean 2 2 Physical Properties of Water Water molecules are asymmetrical, and this has very important consequences. 1. The electric charge is asymmetrical, causing strong attraction between molecules, resulting in: * High melting temperature. * High boiling point. * High heat of vaporization. * High surface tension. 2. The molecule has a large dipole moment, resulting in: * High dielectric constant. * Great power for dissolving inorganic chemicals, which leads to high salinity and conductivity of sea water. 3. The high conductivity causes: * Rapid electrolysis of metals in sea water, causing rapid corrosion. * The motion of sea water in Earth's magnetic field creates a potential. Measurements of the potential can be used for measuring the velocity of oceanic currents. 4. Water molecules pack together either in tetrahedral structures or in spherical, close-packing, structures of ice. * The properties of the tetrahedral structure, which is more common at higher temperatures, is superimposed on the properties of the ice structure,which is more common at lower temperatures. * The conflict between these two structures leads to the parabolic shape of water properties as a function of temperature (Fig. 6.2). 5. Tetrahedral packing is denser than the spherical close packing of ice . * The maximum density of pure water is above the freezing point. * Ice is less dense than water. * The maximum density of sea water, however, is at freezing. Figure 6.2 The shape of the water and ice molecules determines the density. The ice molecule is packed in a lattice that takes more volume than water molecules. Hence water expands on freezing. From Thurman (1985). 3 Potential Temperature, Potential Density, and Sigma As water sinks and flows into the deep ocean, it changes depth and moves far away from its original source at the surface. To trace the movement of water in the deep ocean, we must compare temperature at one depth with temperature at another. This is possible, but it requires calculating changes in temperatures due to compressibility of sea water. As pressure increases, water is compressed, and the compression does work on the water. This causes the water to warm. To understand the warming, consider a cube containing a fixed mass of water. As the cube sinks, its sides move inward as the cube is compressed. Potential Temperature To avoid calculating temperature changes due to compressibility of water, oceanographers (and meteorologists who have the same problem in the atmosphere) use the concept of potential temperature. Potential temperature is defined as the temperature of a parcel of water at the sea surface after it hasbeen raised adiabically from some depth in the ocean. Raising the parcel adiabically means that it is raised in an insulated container so it does not exchange heat with its surroundings. Of course, the parcel is not actually brought to the surface. Potential temperature is calculated from the temperature in the water at depth, the in situ temperature. Density and sigma-t Density is another important property of sea water. Less dense water floats on more dense water, and if we wish to determine how water can move within the ocean, we need to be able to calculate the density of water with an accuracy of a few parts per million. Density of water at the sea surface is typically 1027 kg/m3. For simplification, physical oceanographers often quote only the last 2 digits of the density, a quantity called the density anomaly or Sigma (s,t,p): s(s,t,p) = r(s,t,p)-1000 kg/m3 (6.6) s(s,t,p) is typically 27.00 kg/m3. For studies of the surface layers of the ocean, compressibility can be ignored, and a new quantity sigma-t (written st) is used: st = s(s,t,0) (6.7) This is the density anomaly of a water sample when the total pressure on it has been reduced to atmospheric pressure (i.e. zero water pressure), but the temperature and salinity are in situ values. 4 4b 5 5 6 Important Concepts 1. 2. 3. 4. 5. 6. 7. item Density in the ocean is determined by temperature, salinity, and pressure. Density changes in the ocean are very small, and studies of water massesand currents require density with an accuracy of 10 parts per million. Density is not measured, it is calculated from measurements oftemperature, salinity, and pressure using the equation of state of sea water. Accurate calculations of density require accurate definitions oftemperature and salinity and an accurate equation of state. Salinity is difficult to define and to measure. To avoid the difficulty, oceanographers use conductivity instead of salinity. They measure conductivity and calculate density from temperature, conductivity, and pressure. A mixed layer of constant temperature and salinity is usually found inthe top 1-100 meters of the ocean. The depth is determined by wind speed andthe flux of heat through the sea surface. To compare temperature and density of water masses at different depths inthe ocean, oceanographers use potential temperature and potential density which remove most of the influence of pressure on density. Definition of Temperature Many physical processes depend on temperature; and a few can be used to define absolute temperature T. The unit of T is the kelvin, which has the symbol K. The temperature scale in kelvin T is related to the temperature scale in degrees Celsius t/° C by: t[° C] = T [K] -273.15 (6.5) where the brackets give the units. The practical temperature scale was revised in 1887, 1927, 1948, 1968, and 1990 as more accurate determinations of absolute temperature become accepted. The most recent scale is the International Temperature Scale of 1990 (ITS-90). It differs slightly from the International Practical Temperature Scale of 1968 IPTS-68. At 0° C they are the same, and above 0° C ITS90 is slightly cooler. t90-t68 = -0.002 at 10° C, -0.005 at 20° C, -0.007 at 30° C and -0.010 at 40° C. Notice that while oceanographers use thermometers calibrated with an accuracy of a millidegree, which is 0.001° C, the temperature scale itself has uncertainties of a few millidegreees. 7 8 9 Figure 6.6 Mean sea-surface salinity. Contour interval is 0.25 psu. Shaded areas exceed 36 psu. (FromLevitus, 1982). 9 10 Figure 6.7 Zonal average of sea-surface salinity calculated for all oceans from Levitus (1982) and the difference between evaporation and precipitation (E - P) calculated from data shown in Figure 5.14. The annual range of sea-surface temperature is highest at mid-latitudes,especially on the western side of the ocean (Figure 6.5). In the west, cold airblows off the continents in winter and cools the ocean. The cooling dominates the heat budget. In the tropics the temperature range is mostly less than 2° C.The distribution of sea-surface salinity also tends to be zonal. The saltiest waters are at mid-latitudes where evaporation is high. Less salty waters are near the equator where rain freshens the surface water, and at high latitudes where melted sea ice freshens the surface waters (Figure 6.6. The zonal (east-west) average of salinity shows a close correlation between salinity and evaporation minus precipitation plus river input (Figure 6.7). Figure 6.5 Annual range of sea-surface temperature in °C calculated from the Reynolds and Smith (1995) mean sea-surface temperature data set. Contour interval is 1° C with heavy contours at 4° C and 8° C. Shaded areas exceed 8° C. 4 Because many large rivers drain into the Atlantic and the Arctic Sea, why is the Atlantic saltier than the Pacific? Broecker (1997) showed that 0.32 Sv of the water evaporated from the Atlantic does not fall as rain on land. Instead, it iscarried by winds into the Pacific (Figure 6.8). Broecker points out that the quantity is small, equivalent to a little more than the flow in the Amazon River, but "were this flux not compensated by an exchange of more salty Atlantic waters for less salty Pacific waters, the salinity of the entire Atlantic would rise about 1 gram per liter per millennium.'' Figure 6.8 Water transported by the atmosphere into and out of the Atlantic. Basins draining into the Atlantic are black, deserts are white, and other drainage basins are shaded. Arrows give direction of water transport by the atmosphere, and values are in Sverdrups. Bold numbers give the net transport for the Atlantic. Overall, the Atlantic loses 0.32 Sv, an amount approximately equal to the flow in the Amazon River. From Broecker (1997). 11 11 12 Potential Density For studies of processes deeper within the ocean, compressibility cannot beignored. Because changes in pressure primarily influence the temperature of the water, the influence of pressure can be removed, to a first approximation, by using the potential density}. 14 Potential density is the density a parcel of water would have if it were raised adiabatically to the surface. The potential density anomaly of such a sample is sq (sigma-theta). sq= s(s,q,0) (6.8) Potential density is useful because it removes the primary influence of pressure on density. 1.5 1.7 Table 6.2 Summary of Measurement Accuracy Variable Range Best Accuracy Temperature 42 °C ± 0.001 °C Salinity 1 psu ± 0.02 psu by titration ± 0.005 psu by conductivity Pressure 10,000 dbar ± 0.65 dbar Density 2 kg/m3 ± 0.005 kg/m3 2.0 Figure 6.11 Profiles of in situ and potential temperature and density in the Mindinao Trench in the Pacific: (a,b) vertical profiles, (c,d) vertical sections. (From Pickard and Emery, 1990). 15 15 16 1 Geographical Distribution of Surface Temperature and Salinity The distribution of temperature at the sea surface tends to be zonal, that is, it is independent of longitude (Figure 6.3). Warmest water is near the equator, coldest water is near the poles. The deviations from zonal are small. Equatorward of 40°, cooler waters tend to be on the eastern side of the basin. North of this latitude, cooler waters tend to be on the western side. Figure 6.3 Mean sea-surface temperature calculated from the optimal interpolation technique (Reynolds and Smith, 1995) using shipreports and AVHRR measurements of temperature. Contour interval is1° C with heavy contours every 5° C. Shaded areas exceed 29° C. 2 The anomalies of sea-surface temperature, the deviation from a long term average, are small, less than 1.5° C except in the equatorial Pacific where the deviations can be 3° C (Figure 6.4). Figure 6.4 Sea-surface temperature anomaly for January 1995 relative to mean temperature shown in figure 6.3 using data published by Reynolds and Smith (1995) in the Climate Diagnostics Bulletin for February 1995. Contour interval is 1° C; shaded areas are positive. Mean Temperature and Salinity of the Ocean The mean temperature of the ocean's waters is: T = 3.5° C; and the mean salinity is S = 34.7 psu. The distribution about the mean is small: 50% of the water is in the range: 1.3° C < T < 3.8° C 34.6 psu < S < 34.8 psu The Oceanic Mixed Layer Wind blowing on the ocean stirs the upper layers leading to a thin mixed layer at the sea surface having constant temperature and salinity from the surface down to a depth where the values differ from those at the surface. The magnitude of the difference is arbitrary, but typically the temperature at the bottom of the layer must be no more than 0.02-0.1° colder than at the surface. Note that the both temperature and salinity must be constant in the mixed layer. We will see later that mean velocity can vary with depth in the mixed layer. The mixed layer is roughly 10-200 m thick over most of the tropical and mid-latitude belts (Figure 6.9 upper). The mixed layer also tends to be saltier than the deeper layers except at high latitudes (Figure 6.9 lower). Below the mixed layer, water temperature rapidly decreases with depth. The range of depths where the rate of change, the gradient of temperature, is large is called the thermocline. Because density is closely related to temperature, the thermocline also tends to be the layer where density gradientis greatest, the pyncocline. Figure 6.9 (a) Typical mean temperature profiles in the open ocean. (From Pickard and Emery, 1990). Figure 6.9 (b) Typical mean salinity profiles in the open ocean. (From Pickard and Emery, 1990). The depth and temperature of the mixed layer varies from day to day and from season to season in response to two processes: 1. Heat fluxes through the surface heat and cool the surface waters. Changes in temperature change the density contrast between the mixed layer and deeper waters. The greater the contrast, the more work is needed to mix the layer downward and visa versa. 2. Turbulence in the mixed layer provides the mechanical work necessary to mix heat downward. The turbulence depends on the wind speed and on the intensity of breaking waves. Turbulence mixes water in the layer, and it mixes the water in the layer with water in the thermocline. The mid-latitude mixed layer is thinnest in late summer when winds are weak, and sunlight warms the surface layer. At times, the heating is so strong, and the winds so weak, that the layer is only a few meters thick. In Fall, early storms mix the heat down into the ocean thickening the mixed layer, but little heat is lost. In Winter, heat is lost, and the mixed layer continues to thicken, becoming thickest in late winter. In Spring, winds weaken, sunlight increases,and a new mixed layer forms (Figure 6.10). The mixed layer rarely extends below two hundred meters. Below the upper two hundred meters is a permanent thermocline that merges with the cold, deep waters of the ocean's interior. Figure 6.10 Growth and decay of the seasonal thermocline at Ocean Station "Pappa'' at 50° N, 145° W in theNorth Pacific. (From Pickard and Emery, 1990). The rate of in-situ temperature increase with depth for seawater ranges from about 0.05°C per 1000m at the surface to ~0.15°C per 1000m at 4000m. -use 0.10°C per 1000m as an average rate of adiabatic heating in the ocean The Potential Temperature of water is the temperature the seawater would have if it was moved from its in-situ depth to the surface without any loss or gain of heat, i.e. adiabatically -so the potential temperature of a water parcel that has an in-situ temperature of 0.40°C at 4000m is about 0.00°C 0.40°C - 4000m*(0.10 /1000m) = 0.00°C -this means that the act of moving the water parcel from the surface to 4000m accounted for about 0.4°C of its measured in-situ temperature -Potential Temperature is denoted by symbol theta (q ) -thus q = In-situ Temperature ñ Pressure * (∆temp/∆ Pressure) 4. Why do oceanographers use potential temperature, rather than in-situ temperature, to compare the temperature properties of different water parcels in the deep ocean? -Comparing potential temperature, rather than in-situ temperature, allows one to estimate whether the two water parcels at different depths in the ocean could have had the same temperature when those water parcels were at the surface. Note: To calculate theta (q) we have to measure both in-situ temperature and pressure. -oceanographers do this using a device called a CTD which measures the in-situ conductivity (C) (which is related to salinity), temperature (T) and pressure which can be converted to depth (D) 8 Salinity does not vary monotonically with depth, like temperature does (Fig 11) -salinities, generally, are greater in the surface than at depthóWhy? -overall there is little S variation in the deep sea (about 34.4 to 35 â) -there is a major subsurface salinity minimum at 1000m in the southern oceans -where is the salinity of the deep water similar to that of surface water? -contours of equal salinity called isohalines 4. How does a subsurface salinity minimum (or maximum) occur? -it canít be the result of only vertical mixing between surface and deep water because there is higher S above and below -Is low S at depth caused by in-situ processes? -NO, salinity is neither produced nor consumed once the water parcel moves away from the surface (where evaporation and precipitation affect salinity) -A subsurface minimum (or maximum) in S is caused by currents moving surface water into this depth region -For example, the S minimum at 1000m between 20°S and 50°S is caused by low salinity surface water at about 50-60°S sinking to about 1000m and moving equatorward (this water mass is called Antarctic Intermediate Water) (Fig. 11) Homework Problem Set 1 Answer all questions clearly and completely. Compare temperature to potential temperature. Which is warmer? Why is that ? Where in the water column is the difference greatest? 2. T-S station comparison: 2a. What is the maximum depth the surface mixed layer could be? Don’t forget to explain your answer. 2b. Which station has more variability in temperature? Salinity? Why is there a salinity maximum around 100 m at the southern station ? 3 . Density and stability 2 station comparison: Check the two profiles, one of sq (sq =potential density- 1000 kg/m3) and the other of stability (that is dr/dz) for the two stations. 3a. What is the shallowest depth in the southern station at which you can find water with density the same as the surface mixed layer in the northern station? 3b. Which station is there greater water column stability above 2000m? Which is more likely to experience deep (i.e. greater than 1000m) convection? Study questions: answer in 1-2 sentences Seawater properties 1. What properties of seawater determine its density? 2. What is the pressure at the bottom of the ocean relative to sea surface pressure? What unit of pressure is very similar to 1 meter? 3. What happens to the temperature of a parcel of water (or any fluid or gas) when it is compressed adiabatically? 4. What are the significant differences between freezing pure water and freezing seawater? What happens to the salt in frozen seawater? 5. Fresh water has a density maximum at a temperature above the freezing point, which allows ice to float. Is this also true for sea water? Why does ice formed from sea water float? 6. How deep is a typical mixed layer if mixed by wind? How deep can it reach if driven by cooling? 7. What are the typical vertical temperature and salinity profiles in the subtropical and subpolar regions of the North Pacific? 8. What is the typical number of layers which people use to describe the ocean in general in mid-latitudes? What are the most general names and typical depth ranges of the layers? (Do not name a specific water mass like "North Atlantic Deep Water".) 9. Is the North Atlantic Ocean saltier or fresher on average than the Pacific?








