Nitric Oxide Chemistry - California State University, Long Beach
advertisement
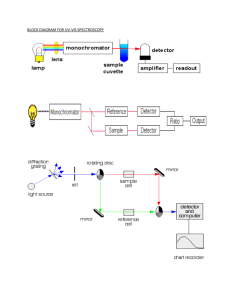
Structural Characterization and Electronic Properties of a Cyclic Non-heme-iron-dinitrosyl Complex: [Fe(NO)2Imidazole]4 Eric Sundberg and Lijuan Li California State University, Long Beach Nitric Oxide Chemistry • 30 Years ago: – A major contributor to smog – An ingredient in cigarette smoke and automobile exhaust – A suspected carcinogen – An ozone depleting agent – Toxic, noxious chemical Koshland, D. E., Jr. Science 1992, 258, 1861-1902 Nitric Oxide Chemistry • In 1992, it was named the “Molecule of the Year” by the journal Science in an article titled, “NO News is Good News” • In 1998 three gentlemen were awarded the nobel prize in medicine and physiology for their work involving the biological aspects of nitric oxide Nitric Oxide Chemistry Furchgott Ignarro Murad Discovered that nitric oxide works as a signaling molecule in the cardiovascular system http://www.nobel.se/medicine/laureates/1998/index.html Nitric Oxide Chemistry Numerous Physiological Functions • Maintains blood pressure by dilating blood vessels • Kills foreign invaders • Involved in neurotransmission • Involved in learning and memory processes • Controls platelet aggregation • Stimulates penile erections Feldman, P. L.; Griffith, O. W.; Stuehr, D. J. Chem Eng. News 1993, 71 (Dec), 26-38 Heme Versus Non-heme • Nitrosyl (NO) on a hemoglobin molecule • Nitrosyl attached to a non-heme molecule CH3 + NO NO CH3 N N NO Fe N Fe N CH3 CH3 NO L Fe COOH COOH L NO Muller, B.; kleschyov, A. L.; Stoclet, J. C. Br. J. Pharm. 1996, 119, 1281-1285 Outline Non-heme iron Dinitroyls Synthesis Characterization FT-IR X-ray Crystallography Classification EPR and NMR Stability UV-vis Electrochemistry Density Functional Theory Synthesis Fe(CO)2(NO)2 + C3H4N2 O O N N NH Fe C O C O Starting Material: Dicarbonyldinitrosyliron(0) N Ligand: Imidazole Synthesis • After 2 Hours: O • After 24 Hours: O N N Fe N HN C O N 80 C N %T 1 00 Fe C O O 60 O 60 N Fe N 1731.01 %T N C 40 HN 1686.63 O 1982.50 Fe (CO)(NO)2(Im) 80 1757.57 O 1806.83 O 2031.69 Fe (CO)2(N O)2 2088.01 Characterization O 20 %T 1796.78 20 1725.95 Fe (NO)2(I m)2 15 10 2 600 2 400 2 200 2 000 W ave num bers (c m-1 ) 1 800 1 600 Characterization • X-ray Crystallography shows a cyclic compound with an acetone molecule trapped inside Materials and Methods • Recrystallized [Fe(NO)2Im]4 • Nuclear Magnetic Resonance – Dissolved 50mg of sample into 2 mls of deuterated THF and transferred to a NMR tube – AC200 Spectrometer • Electron Paramagnetic Resonance – Dissolved 2 mg of sample into degassed 1 ml of THF and transferred to EPR tube – Brooker EMX Model EPR Spectrometer Materials and Methods • Electrochemistry – 3 electrode system with a gold working electrode – THF was used as the solvent – Scan Rate of 100 mV sec-1 – 10mM [Fe(NO)2(Imidazole)]4 solution and a 0.1M electrolyte solution, (tetrabutylammonium perchlorate) – Computer Controlled Electrochemical Analyzer Autolab Electrochemical Insturments http://www.ecochemie.nl/ Materials and Methods • UV-vis Spectroscopy – Varian 300 Bio UV-vis spectrophotometer – Dilution Series in THF: 0.1-0.8 mM Cary Uv-vis Spectrometer www.varianinc.com Materials and Methods • Stability – FT-IR spectroscopy (Nicholet 380 Spectrometer) – 0.2mM in THF at 15 and 30 minutes • Spin Density Functional Theory – Performed using X-ray crystallographic coordinates – Dr. Fu-Ming Tao at Cal. State Univ. Fullerton Nuclear Magnetic Resonance 1H H1 NMR (H2&H3) (H1) 9.0 H2&H3 8.0 7.0 6.0 5.0 4.0 ppm 3.0 2.0 1.0 0 Nuclear Magnetic Resonance 13C C1 NMR C2&C3 C2&C3 C1 240 210 180 150 120 ppm 90 60 30 Electron Paramagnetic Resonance g=2.03 20G Hyperfine Structure Electrochemistry UV-vis Spectroscopy MLCT d-d transitions Stability under Oxygen Fe (NO)2(I m )2 23 22 21 20 19 17 1796.78 16 15 14 13 12 11 1725.95 %Transmi ttance 18 10 9 3 400 3 200 3 000 2 800 2 600 2 400 2 200 W ave num bers (c m -1 ) 2 000 1 800 1 600 1 400 1 200 Spin Density Functional Calculations • Will provide wavefunction analysis of the molecule – = a + b + c… – A diagram of the electron density on the molecule N 80 C N %T 1 00 Fe C O O 60 O 60 N Fe N 1731.01 %T N C 40 HN 1686.63 O 1982.50 Fe (CO)(NO)2(Im) 80 1757.57 O 1806.83 O 2031.69 Fe (CO)2(N O)2 2088.01 Spin Density Functional Calculations O 20 %T 1796.78 20 1725.95 Fe (NO)2(I m)2 15 10 2 600 2 400 2 200 2 000 W ave num bers (c m-1 ) 1 800 1 600 Spin Density Functional Calculations SingleSubstituted Product N: Fe N: Fe :N O :N O HN Cyclic Product N Fe + Conclusion • [Fe(NO)2Im]4 has been characterized by – X-ray Crystallography and FT-IR Spectroscopy – NMR and EPR techniques • Electrochemistry showed 2 chemically reversible reductions • UV-vis spectroscopy showed MLCT and dd orbital transitions Conclusion • Low stability of compound in solution when exposed to oxygen • Spin Density Functional Theory showed excess electronic density on the imidazole • Potential Medicinal Applications Future Studies • Mass spectrometry • Elemental analysis • NMR studies of decomposed sample • Laser flash photolysis Acknowledgements • Dr. Lijuan Li • Dr. Ximeng Wang • The Beckman Scholars Foundation • Howard Huges Medical Institute