PPT
advertisement
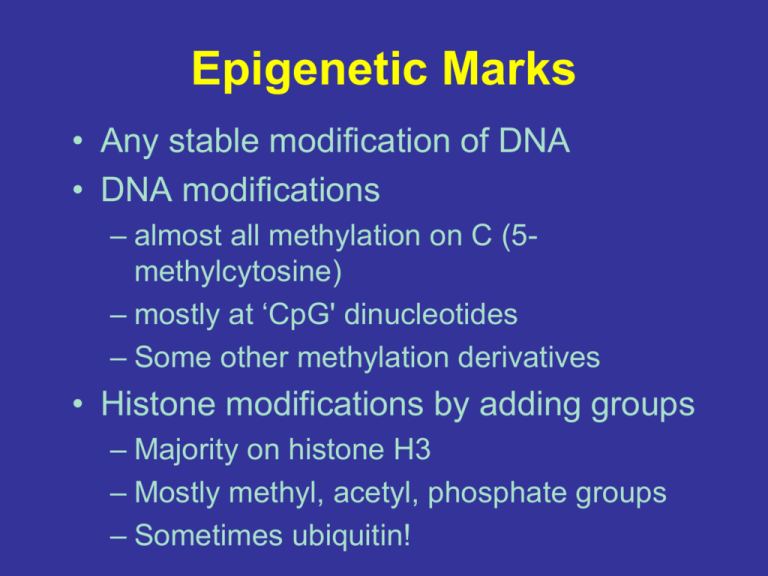
Epigenetic Marks • Any stable modification of DNA • DNA modifications – almost all methylation on C (5methylcytosine) – mostly at ‘CpG' dinucleotides – Some other methylation derivatives • Histone modifications by adding groups – Majority on histone H3 – Mostly methyl, acetyl, phosphate groups – Sometimes ubiquitin! Nucleosomes and Histone Marks • DNA wrapped around nucleosomes (8 histones) – ~142 bases wrapped twice • Nucleosomes may be consistent (‘well positioned’) from cell to cell at key regulatory sites CpG Islands and Promoters • Although C & G constitute 42% of the human genome, less than 1% of pairs are CpG – Less than ¼ of expected frequency of 0.04 • A ‘CpG island’ is a run of “CpG-rich” sequence – Typically taken as > 2% of pairs – This definition is not precise (there are others) • Many CpG islands occur within promoters • In vertebrates >70% of CpG cytosines methylated • Methylation mediated by DNA methyltransferases Image: cellscience / alcat.com Epigenetic Marks at Promoters • H3K4me3 typically marks active promoters (and a few other places) • H3K27ac typically marks active regulatory sites including promoters • H3K27me3 marks repressed promoters – Typically developmental genes • DNA methylation dips at silenced promoters • One histone is kicked out at active promoters Central Dogma: DNA to RNA to Protein Processing / Translocation Translation How to use an mRNA to create a protein? NOT OBVIOUS – RNA can’t hydrogen bond with the 20 different amino acids in a meaningful way One four base code must be translated into another 20 amino acid code Energy intensive process that requires: • mRNA • tRNA • aminoacyl tRNA synthetases • ribosomes tRNAs serve as molecular adaptors between amino acids and codons • 75 – 95 NT • exact sequence varies • 3’ terminus is the AA attachment site • most tRNAs can recognise >1 codon • contains both paired and unpaired regions Many types, all with same basic structure, each attaching a specific amino acid and recognizing specific codons tRNAs contain several non-standard bases Also thymine, hypoxanthine, methylguanine, inosine • All result from post-transcriptional modification of bases • Improves tRNA function • Some (hypoxanthine) involved in codon recognition tRNAs assume a characteristic “L” shaped 3D conformation Tertiary structure stabilised by: • Non-Watson-Crick base pairings between bases • Base-sugar phosphate interactions • Base stacking in base-paired regions Aminoacyl tRNA synthetases “charge” tRNAs Amino acids are first adenylylated (AMP transferred) tRNA charging Second step results in release of AMP and charging of tRNA by attachment of AA to either 2’ or 3’ OH of tRNA It is an underappreciated fact that there is an implicit second “structural” genetic code • Most tRNA synthetases interact with several different “isoaccepting” tRNAs Although there can be many tRNAs for a particular amino acid, ONLY ONE aminoacyl tRNA synthetase is responsible for attaching a particular amino acid to all of the appropriate tRNAs. The anticodon loop and a 3’ “discriminator” base are key for recognition and specificity Aminoacyl tRNA synthetases discriminate between structurally similar amino acids Free energy differences of only 2-3 kcal/mol are sufficient to discriminate 99% of the time, however the actual error rate is 10x less…. Co-crystal stucture of a tRNA synthetase with its cognate tRNA 3’ acceptor and discriminator adjacent to the adenylylated AA Contact with the anticodon The “universal” genetic code Dissimilar anticodons can encode the same AA Mitochondria and some other organisms use a slightly modified code Many amino acids have more than one cognate tRNA However, there is NOT a separate tRNA for every possible codon….. The “Wobble” hypothesis Non-standard base pairing increases the codon repertoire of many tRNAs Inosine promiscuously base pairs with C, U, or A G-U base pairing can also occur tRNA 3D structure suggests why wobble is restricted to the third position The 5’ anticodon position is less hindered by base stacking interactions The genetic code explains the outcome of various types of mutations • Insertion or deletion of a base results in a “frameshift” mutation • Results in incorporation of incorrect amino acids • Frameshifts can also cause premature termination Single base pair mutations • Usually result in a “missense” mutation…… Single base pair mutations …or, if a stop codon is created, a “nonsense” mutation Mutations can sometimes be suppressed by a second mutation Site of previous base deletion leading to frameshift Second mutation correct the frameshift This is an example of intragenic suppression Suppression can also be intergenic Mutated gene Mutated gene and nonsense suppresor In intergenic suppression, a mutation in a second gene compensates for a mutation in the first Translation can proceed in three possible reading frames Open Reading Frames (ORFs) corresponding to possible peptides occur between predicted start and stop codons The ribosome is a complex multisubunit ribonucleoprotein Note: Eukaryotic ribosome has 60S and 40S subunits, overall size is 80S • Over 50 proteins • Three rRNAs • Huge 2.5 MDalton complex • Organised into large and small subunits The ribosome is a complex multisubunit ribonucleoprotein 50S subunit tRNA pocket 30S subunit The eukaryotic ribosome Note that S, the Svedburg coefficient, does not measure mass and is not additive The prokaryotic ribosome The ribsomes of eukaryotic organelles are similar to prokaryotic ribosomes In prokaryotes, transcription and translation are coupled NOT true in eukaryotes – translation occurs in the cytoplasm, not the nucleus Overview of the translation cycle Proteins are synthesised in the N to C direction Multiple ribosomes can simultaneously associate with a single mRNA. Such aggregates are known as polyribosomes or simply polysomes Chain growth occurs via the peptidyl transferase reaction • This is a coupled reaction – energy ultimately comes from ATP hydrolysis during tRNA charging • The nascent polypeptide is always tethered to a tRNA Not all ORFs get translated! In prokaryotes, the Shine-Dalgarno sequence, also known as the Ribosome Binding Site (RBS) interacts with the 16S rRNA to recruit the translational machinery • Sometimes a second RBS is not required in polycistronic messages when overlap occurs (e.g. 5’ – AUGA – 3’) Eukaryotes use different signals to recruit translation machinery Eukaryotic ribosomes associate with the 5’ mRNA cap and “scan” in a 5’-3’ direction for start codons (AUG) The “Kozak” sequence, if present, increases the efficiency of translation initiation The ribosome does not participate in proofreading Chemical reduction (in vitro) The ribosome is perfectly happy to incorporate the WRONG amino acid into a growing peptide chain… Proofreading happens at the level of the aminoacyl tRNA synthetases, which have a proofreading pocket. Study Question 11 Degeneracy and frequency of amino acids Most common Leu Gly Ser Least common Trp Met His Study Question 12 Single mutation from AGA Silent: | Hydrophilic/ Hydrophilic: | Study Question 12 Single mutation from AGA Silent: | Conservative: | Hydrophilic/ Hydrophilic: || Hydrophilic/ Hydrophobic: | Study Question 12 Single mutation from AGA Silent: || Conservative: | Hydrophilic/ Hydrophilic: ||| Hydrophilic/ Hydrophobic: | Other: | Study Question 8 Why do introns exist? AAAAAA...AAA Splicing AAAAAA...AAA Splice boundaries highly conserved Study Question 8 Why do introns exist? Protein #1 hormone responsiveness Protein #2 protein kinase DNA binding chromosomal rearrangement Hormone-responsive protein kinase DNA-binding protein Study Question 8 Why do introns exist? hormone responsiveness protein kinase DNA binding AAAAAA...AAA New protein: Hormone-responsive DNA-binding protein Nucleic Acids complementarity Study Question 4 Example of palindromic DNA Study Question 5 Analogy: Translation / Tape recorder





