Balancing Chemical Equations
advertisement

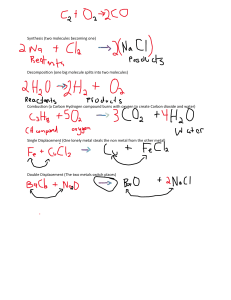
Chemistry CH 8 Reading Assignment Pg 261-274 1. Define chemical reaction: 2. The starting compounds of a chemical reaction are known as ___ and the resulting compounds are known as ____. 3. What does the Law of Conservation of Mass state: 4. What does a chemical equation state: 5. What does the following represent in a chemical reaction 4(NH4)2Cr2O7 (s) a. 4 b. (NH4)2Cr2O7 c. (s) d. 6. Name four evidence that a chemical reaction has occurred: a. b. c. and what is a precipitate (ppt) d. 7. State the diatomic molecules: 8. What is a coefficient and what does it represent? 9. What is a word equation and write the word equation for the following reaction: Zn + Pb(NO3)2 Zn(NO3)2 + Pb 10. What is a formula equation and how does it differ from a chemical equation? 11. Write the formula equation for the following: solid zinc sulfide reacts with oxygen gas to produce solid zinc oxide and sulfur dioxide gas. 12. Write the chemical equation for the above word equation: 13. Know the symbols on Table 2 page 266. 14. What is a catalyst and how can it be represented in a chemical equation? 15. What is a reversible reaction and how is it expressed? 16. How can the equation 2Cl2(g) + 4KBr(aq) 4KCl(aq) + 2Br2(g) be properly expressed and what does (aq) mean? 17. Does having a chemical equation indicate that the reaction actually occurs? 18. Some steps to remember when balancing chemical equations: a. Balance from left to right and one atom at a time b. Never change subscripts, only add coefficients in front of the compounds c. If polyatomic ions stay the same on both sides of the equation, treat them as if they are just a single unit d. Balance H and O last e. Check to make sure it is balanced and reduce coefficients if possible Pg 276-284 19. What is a synthesis reaction, what is the general equation, and how can use recognize if a synthesis reaction will occur? 20. Write the synthesis reaction for Iron III reacts with oxygen: 21. What is a decomposition reaction, what is the general equation, and how can use recognize if a decomposition reaction will occur? 22. You will need to memorize the five types of decomposition reactions: a. How will a binary compound decompose? b. What is electrolysis? c. How will a metal carbonate decompose and what is usually needed for this reaction to occur? d. How will a metal hydroxide decompose? e. How will a metal chlorate decompose? f. How will an acid decompose? 23. What is a single displacement reaction, what is the general equation, and how can use recognize if a single displacement reaction will occur? 24. What will a metal displace in the compound it is reacting with? 25. What will a nonmetal displace in the compound it is reacting with? 26. Know that water reacts by H+ and OH27. What is a double displacement reaction, what is the general equation, and how can use recognize if a double displacement reaction will occur? 28. What is a precipitate? 29. What is a combustion reaction, what is the general equation, and how can use recognize if a combustion reaction will occur? 30. What is a hydrocarbon? 31. In a combustion reaction, what will the hydrocarbon react with and what two products are ALWAYS produced? 32. You should be able to predict the products of the reactions above. Pg 285-287 Honors Chemistry only 33. 34. 35. 36. 37. What is the activity series and what can it help you do? What makes a metal and nonmetal more reactive than another metal and nonmetal? The activity series is used to predict single displacement reactions, how does it work? Know how to use the activity series! Memorize the solubility table given to you in class to help predict if a double displacement reaction will occur.