CHEMISTRY - Special Measurement Problems NAME:
advertisement

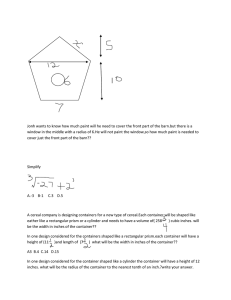
CHEMISTRY - Measurement Review Problems DO NOT HAND THIS IN! 1. Complete these conversions: (a) 8050 kg = _____ pounds (b) 0.654 hL = _____ quarts (c) 7,000,000 mm = _____ yards (d) 44.6 gallons = _____ nL 2. A block of aluminum has a density of 2.8 g/cm3. Its dimensions are 4 inches x 2 inches x 8 inches. What does it weigh? 3. Perform these operations: (a) (6 x 103)4(5.8 x 10-4)3 = (b) (2.54 x 10-3)[(7.7 x 102) + (8.44 x 104)]2 = 4. A rectangular chunk of an unknown material has a mass of 6 kg and has a volume of 8 x 106 mL. Will it float in water? 5. A speed of 15 feet/second is how many cm/hour? 6. A liquid has a volume of 3.5 liters and weighs 88.5 dg. What is its density in mg/mL? 7. How many square cm are in 120 square meters? 8. The density of a rock does not change when it is submerged in water, but your density changes you are submerged! Why is this so? 9. Round these numbers to THREE SIGNIFICANT DIGITS: (a) 71.088233 (b) 0.0000515151 (c) 1,431,567 10. The mass of an empty container is 88.25 g. The mass of the container when filled with a liquid (density = 1.25 g/mL) is 150.50 g. What is the volume of the container?