CHEMISTRY - Special Measurement Problems NAME:
advertisement
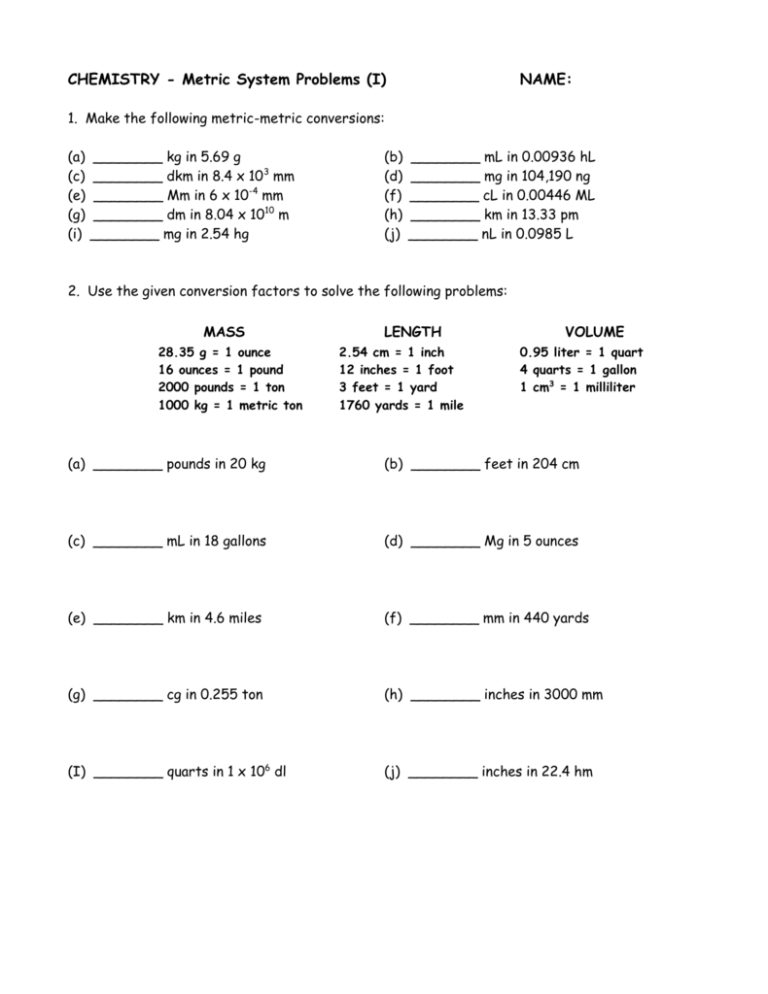
CHEMISTRY - Metric System Problems (I) NAME: 1. Make the following metric-metric conversions: (a) (c) (e) (g) (i) ________ kg in 5.69 g ________ dkm in 8.4 x 103 mm ________ Mm in 6 x 10-4 mm ________ dm in 8.04 x 1010 m ________ mg in 2.54 hg (b) (d) (f) (h) (j) ________ mL in 0.00936 hL ________ mg in 104,190 ng ________ cL in 0.00446 ML ________ km in 13.33 pm ________ nL in 0.0985 L 2. Use the given conversion factors to solve the following problems: MASS 28.35 g = 1 ounce 16 ounces = 1 pound 2000 pounds = 1 ton 1000 kg = 1 metric ton LENGTH 2.54 cm = 1 inch 12 inches = 1 foot 3 feet = 1 yard 1760 yards = 1 mile VOLUME 0.95 liter = 1 quart 4 quarts = 1 gallon 1 cm3 = 1 milliliter (a) ________ pounds in 20 kg (b) ________ feet in 204 cm (c) ________ mL in 18 gallons (d) ________ Mg in 5 ounces (e) ________ km in 4.6 miles (f) ________ mm in 440 yards (g) ________ cg in 0.255 ton (h) ________ inches in 3000 mm (I) ________ quarts in 1 x 106 dl (j) ________ inches in 22.4 hm CHEMISTRY – Metric System Problems (II) NAME: 1. The density of mercury is 13.6 g/mL. How much volume will 300 g occupy? How many kg would there be in 2.22 liters of mercury? 2. The density of pure silver is 10.5 g/cm3. If 5.25 g of pure silver pellets are added to a graduated cylinder containing 11.2 mL of water, to what volume level will the water in the cylinder rise? 3. Find the density in g/cm3 of 4000 milliliters of a liquid that has a mass of 3050 g. 4. What is the density of a piece of cork that has a mass of 0.650 g and a volume of 2.71 cm3? 5. If the density of gasoline is 0.68 g/cm3, what is the weight of 10 gallons of gas? 6. A sample containing 33.42 g of metal pellets is poured into a graduated cylinder initially containing 12.7 mL of water, causing the water level in the cylinder to rise to 21.6 mL. Calculate the density of the metal. 7. An aquarium measures 16 inches by 8 inches by 10 inches. How many liters of water does it hold? How many gallons? How many kg of water? 8. The density of silver is 10.5 g/cm3 at 20oC. What happens to the density of a 68 g bar of silver if the bar is cut in half? What happens to the density if the bar is warmed to a high temperature? 9. For a pharmacist dispensing pills or capsules it is often easier to weigh the medication to be dispensed rather than to count the individual pills. If a single antibiotic capsule weighs 0.65 g, and a pharmacist weighs out 15.6 g of capsules, how many capsules have been dispensed? 10. The element bismuth has a density of 9.80 g/cm3. What is the mass of 1.5 liters of bismuth?







