Simultaneous determination of Arbutin, Kojic acid and
advertisement

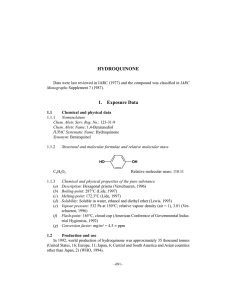
Simultaneous determination of arbutin, kojic acid and hydroquinone in skin lightening cosmetics by MEKC Yi-Hui Lin1, Shou-Mei Wu1,2,3* 1Graduate Institute of Pharmaceutical Science, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan 2Faculty of Fragrance and Cosmetics, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan 3Faculty of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung 807, Taiwan Abstract A simple method of micellar electrokinetic capillary electrophoresis (MEKC) was established for simultaneous analysis of arbutin, kojic acid and hydroquinone. Untreated fused-silica capillary was operated using phosphate buffer (20 mM, pH 6.5) under 20 kV and detection at 200 nm. Baseline separation was attained within 9 minutes. The quantitative ranges were 20-200 μg/mL for arbutin, 20 -100μg/mL for kojic acid and 8-80μg/mL for hydroquinone with correlation coefficients ≥ 0.9994. The R.S.D. and R.E. were all less than 3.0 % for the intra-day and inter-day analysis, and all recoveries were greater than 99 %. The limits of detection was arbutin 5.4 μg/mL, kojic acid 7.1μg/mL and hydroquinone 2.2 μg/mL (S/N = 3, hydrodynamic injection 5 sec). Our method was applied to determine the quality of commercial cosmetic. Assay results were all within the labeled amount of 99.6-102.5%. 2 Introduction Arbutin (AR) HO O O OH OH OH OH 4-hydroxyphenyl-β-D-glucopyranoside extracted from leaves of common bearberry It can compete with L-dopa for receptor site on tyrosinase and hinders the oxidation of L-dopa to L-dopaquinone, thus, can inhibit the formation of eumelanin and phaeomelanin often used in skin care products as a lightening agent 3 Introduction Hydroquinone (HQ) OH HO 1,4-benzenediol It can produce reversible depigmentation of the skin by suppression of melanocyte metabolic processes 4 Introduction Kojic acid (KA) O OH HO O 5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one a natural substance produced by fungi or bacteria, such as Aspergillus, Penicillium or Acetobacter spp It is able to inhibit the creolase and catecholase activities of tyrosinase. can be used in cosmetics to prevent serious sunburning caused by an accumulation of melanin 5 Introduction According to Department of Health, Taiwan, R.O.C Ingredients Limit 1、Kojic Acid (5-Hydroxy-2-Hydroxymethyl-4HPyran-4-one) 2% 2、Arbutin (4-hydroxyphenyl-β-D-glucopyranoside) 7% It also recommended that HQ should be used under prescription because long term used of high concentration hydroquinone (over 5 %) may cause severe side effects including dermatitis, erythema, leukoderma, burning and hyperpigmentation. 6 Aim of this study To develop a rapid, sensitive and quantitative assay for the simultaneous determination of AR, KA and HQ by capillary electrophoresis. Optimization, validation and application of this method were investigated. 7 Materials • • • • • • AR (98%) KA HQ Urea (I.S.) Sodium dodecyl sulfate NeoStrata® pigment lightening gel (Princeton, NJ, USA) • Shiseido® white lucent (Tokyo, Japan) • Grape® quincare ointment (Chung-Li, Taiwan) 8 CE System • A Beckman P/ACE System 2200 equipped with a filter UV detector • The fused-silica capillaries: I.D.= 50 µm; Lt= 57.0 cm; Ld= 50.0 cm • Phosphate buffer containing SDS • Applied voltage: 20 kV • Temperature: 25 ˚C • Wavelength for detection: 200 nm • Hydrodynamic injection pressure: 50 mbar, 5 sec 9 Results and discussion 10 A 0.006 C B D AR+HQ HQ AR HQ KA+HQ KA absorbance AR 0.004 AR KA KA 0.002 0.000 5 6 7 5 6 7 5 6 7 5 6 7 time (min) Figure 1. Effects of SDS concentrations on the migration of AR, KA and HQ each at 200 μM. Electropherograms: (A) overlapping peaks from separation in the absence of SDS, (B) with 60 mM SDS, (C) with 80 mM SDS, (D) with 100 mM SDS. 11 Table 1. Regression analysis for the determination of AR, KA and HQ. Analysis intra-daya AR KA HQ inter-dayb AR KA HQ Regression equation Coefficient of correlation (r) Y=(5.2316±0.0166)X+(0.0149±0.0020) Y=(6.0875±0.0336)X+(0.0023±0.0046) Y=(11.469±0.0249)X+(0.0116±0.0032) 0.9998 0.9994 0.9998 Y=(5.2295±0.0516)X+(0.0109±0.0053) Y=(6.0724±0.1173)X+(0.0018±0.0053) Y=(11.3938±0.1129)X+(0.0009±0.007) 0.9997 0.9996 0.9998 * Concentration ranges for the intra- and inter-day analysis: AR: 0.02-0.2 mg/ml KA: 0.02-0.1 mg/ml HQ: 0.008-0.08 mg/ml a The regression equations of intra-day analysis were calculated from the assay values of prepared standards on a single day (n=3). b The regression equations of inter-day analysis were calculated from the assay values of prepared standards on a single day (n=5). 12 Table 2. Precision and accuracy for the determination of AR, KA and HQ. Concentration Concentration RSD RE know found (mg/ml) (mg/ml) (%) (%) 0.05 0.0504±0.0006 1.20 0.86 0.09 0.0904±0.0004 0.48 0.41 0.16 0.1609±0.0007 0.43 0.57 0.03 0.0299±0.0006 2.03 -0.34 0.07 0.0697±0.0008 1.13 -0.41 0.09 0.0891±0.0007 0.77 -0.95 0.015 0.0150±0.0001 0.57 0.01 0.03 0.0292±0.0005 1.75 -2.63 0.07 0.0698±0.0007 1.05 -0.23 0.05 0.0503±0.0010 2.05 0.70 0.09 0.0902±0.0009 1.04 0.28 0.16 0.1606±0.0010 0.61 0.35 0.03 0.0296±0.0008 2.59 -1.21 0.07 0.0693±0.0009 1.28 -0.96 0.09 0.0893±0.0009 1.05 -0.75 0.015 0.0150±0.0001 0.87 -0.14 0.03 0.0292±0.0006 2.01 -2.80 0.07 0.0701±0.0008 1.17 0.08 Intra-day analysis (n=3) AR KA HQ Inter-day analysis (n=5) AR KA HQ 13 Table 3. Recovery of AR, KA and HQ added to the commercial formulation Concentration Concentration Recovery spiked found (mg/ml) (mg/ml) (%) – 0.0303±0.0002 – 0.02 0.0512±0.0008 104.55 0.07 0.1017±0.0015 102.00 0.16 0.1928±0.0012 101.55 – 0.0222±0.0009 – 0.02 0.0421±0.0010 99.44 0.04 0.0631±0.0002 102.16 0.07 0.0924±0.0005 100.30 – 0.0109±0.0002 – 0.02 0.0313±0.0002 101.9497 0.04 0.0511±0.0002 100.3522 0.06 0.0720±0.0005 101.6940 AR KA HQ 14 Table 4. Quantification of AR, KA and HQ in marketing cosmetics Samples (Labeled amount) AR (3%) KA (2%) HQ (20mg/g) Amount found 1 3.00 2 2.99 3 3.01 Mean 3.00 SD 0.0068 1 2.01 2 2.05 3 2.04 Mean 2.03 SD 0.0152 1 19.94 2 20.27 3 20.35 Mean 20.19 SD 0.1778 15 Conclusions This method can be used for the simultaneous determination of arbutin, kojic acid and hydroquinone and was successfully applied to the content assays of skin whiting cosmetics. 16