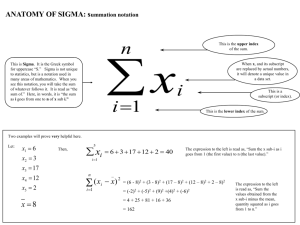
A simplified and robust protocol for immunoglobulin
advertisement

Supplementary Materials for: A simplified and robust protocol for immunoglobulin expression in Escherichia coli cell-free protein synthesis systems Qi Cai+, Jeffrey A. Hanson+, Alexander R. Steiner, Cuong Tran, Mary Rose Masikat, Rishard Chen, James F. Zawada, Aaron K. Sato, Trevor J. Hallam, and Gang Yin* Sutro Biopharma, Inc. 310 Utah Avenue, Suite 150, South San Francisco, CA 94080 + These authors contributed equally to this work. * Correspondence to: Gang Yin; Email: gyin@sutrobio.com 1 Contents Stock solutions for cell-free reactions................................................................................................ 2 Table S1: Detailed cost calculations .................................................................................................. 3 Figure S1: Representative 14C gel for cell-free IgG quantification................................................. 5 Figure S2: Time course of Trastuzumab expression using conventional and simplified protocols ............................................................................................................................................... 6 Stock solutions for cell-free reactions Though most of the reaction components can be added directly in powdered form to the master mix solution, for cell-free reactions described in the manuscript, stock solutions of each compound were made for the present work to facilitate titration of the individual reaction components. The amino acid tyrosine was prepared at 50 mM at pH 11 using KOH due to its low solubility at neutral pH. A 25x amino acid stock solution containing 50 mM each of the remaining 19 amino acids was prepared and had a pH of 5.2. A 50x nucleotide stock solution was created with 60 mM ADP and 43 mM each of GMP, UMP and CMP without adjustment to the pH. A 1 M phosphate solution was prepared at pH 7 with 615 mM K2HPO4 and 385 mM KH2PO4. The following stock solutions were made in milliQ water without adjustments to the pH: 2 M sodium pyruvate, 5 M potassium glutamate, 500 mM magnesieum glutamate, 1 M ammonium glutamate, 33 mM NAD, 80 mM coenzyme A, 1 M potassium oxalate, 3.4 mg/mL folinic acid, 100 mM oxidized glutathione (GSSG), 80 mM hemimagnesium glutamate, 200 mM spermidine, and 200 mM putrescine. Total E. coli tRNA was resuspended at 17 mg/mL. T7 RNA polymerase was produced in house and stored in 50 mM Tris (pH 7.8) 10% sucrose, 300 mM NaCl, 2 mM DTT and 0.1 mM EDTA at -80 °C at concentrations of 5-10 mg/mL. Plasmid DNA was prepared by maxiprep (Qiagen) and resuspended in water at concentrations of 0.2 or 1 mg/mL. The pH of the final master mix solution is 6.7. 2 Table S1: Detailed cost calculations Conventional protocol Reagent Folinic acid E. coli total tRNA Vendor catalog # Sigma 47612-1G Roche Diagnostics 10109550001 unit price 1g 572 0.5g 587 M.W. Conc 0.034g/L $/L % Conc $/L % 19.45 2.94 0g/L 0.00 0.00 0mg/L 0.00 0.00 0.00 0.00 0.00 0.00 0.67 1.60 0.17mg/L 199.58 30.17 Transription Simplified protocol 219.03 33.11 L-Glutamic Acid, Ammonium Salt L-Glutamic acid hemimagnesium salt tetrahydrate MP Biomedicals 02180595.1-100g 100g 77 164.2 10mM 1.26 0.19 Sigma 49605-250G L-Glutamic acid monopotassium salt monohydrate Potassium oxalate monohydrate Sigma G1501-1KG 250g 53.9 1000g 243.5 Sigma 223425 12000g Sodium pyruvate Sigma P2256 β-Nicotinamide adenine dinucleotide hydrate Sigma Coenzyme A sodium salt hydrate 388.61 8mM 0.67 0.10 8mM 203.23 130mM 6.43 0.97 260mM 681 184.23 4mM 0.04 0.01 4mM 0.04 0.10 500g 789.5 110.04 33mM 5.73 0.87 0mM 0.00 0.00 N1636 1g 426.5 663.43 0.33mM 93.37 14.11 0mM 0.00 0.00 Sigma C3144-1G 1g 1480 767.53 0.27mM 306.70 46.36 0mM 0.00 0.00 K2HPO4·3H20 Sigma P5504 10000g 879 228.23 9.2mM 0.18 0.03 9.2mM 0.18 0.44 KH2PO4 Sigma P5379 10000g 454.5 136.09 5.8mM 0.04 0.01 5.8mM 0.04 0.09 Energy-related 0mM 414.44 62.65 12.87 30.76 13.80 32.99 Putrescine MP Biomedicals 0210044180-100g 100g 63.35 88.15 1mM 0.06 0.01 0mM 0.00 0.00 Spermidine Sigma S2626 25g 466 145.25 1.5mM 4.06 0.61 1.5mM 4.06 9.71 4.12 0.62 4.06 9.71 4.68 0.71 4.68 11.19 4.68 0.71 4.68 11.19 Polyamines L-Glutathione oxidized , GSSG Sigma G4376 1000g 3820 612.6 2mM Folding 2mM AMP GMP Sigma A1752 25g 210.5 347.22 1.2mM 3.51 0.53 1mM 3.51 8.39 Sigma G8377 100g 790 407.18 0.86mM 2.77 0.42 1mM 2.77 6.61 UMP Sigma U6375 10g 135.5 369.15 0.86mM 4.30 0.65 1mM 4.30 10.28 CMP Sigma C1006 5g 103 367.16 0.86mM 6.50 0.98 1mM 6.50 15.55 L-Alanine Sigma A7627 1000g 450.5 89.09 2mM 0.08 0.01 2mM 0.08 0.19 L-Arginine Sigma A5006 1000g 219 174.2 2mM 0.08 0.01 2mM 0.08 0.18 L-Asparagine Sigma A0884 1000g 384.5 132.12 2mM 0.10 0.02 2mM 0.10 0.24 L-Aspartic Acid Sigma A7219 1000g 203 133.1 2mM 0.05 0.01 2mM 0.05 0.13 3 L-Cysteine Sigma C7352 1000g 601 121.16 2mM 0.15 0.02 2mM 0.15 0.35 L-Glutamine Sigma G8540 1000g 445 146.15 2mM 0.13 0.02 2mM 0.13 0.31 Glycine Sigma G7126 25000g 914 75.07 2mM 0.01 0.00 2mM 0.01 0.01 L-Histidine Sigma H8000 1000g 552 155.16 2mM 0.17 0.03 2mM 0.17 0.41 L-Isoleucine Sigma I2752 1000g 742 131.18 2mM 0.19 0.03 2mM 0.19 0.47 L-Leucine Sigma L8000 1000g 411 131.18 2mM 0.11 0.02 2mM 0.11 0.26 L-Lysine·H20 Sigma L7039 25000g 3005 182.65 2mM 0.04 0.01 2mM 0.04 0.10 L-Methionine Sigma M9625 1000g 274 149.21 2mM 0.08 0.01 2mM 0.08 0.20 L-Phenylalanine Sigma P2126 1000g 425.5 165.190 2mM 0.14 0.02 2mM 0.14 0.34 L-Proline Sigma P0380 5000g 2180 115.13 2mM 0.10 0.02 2mM 0.10 0.24 L-Serine Sigma S4311 1000g 799 105.09 2mM 0.17 0.03 2mM 0.17 0.40 L-Threonine Sigma T8625 1000g 818 119.12 2mM 0.19 0.03 2mM 0.19 0.47 L-Tryptophan Sigma T0254 1000g 573 204.23 2mM 0.23 0.04 2mM 0.23 0.56 L-Tyrsosine Sigma T8566 1000g 506 181.190 1mM 0.09 0.01 1mM 0.09 0.22 L-Valine Sigma V0500 1000g 382.5 117.15 2mM 0.09 0.01 2mM 0.09 0.21 Building Blocks 19.29 2.92 Total 661.56 19.29 46.12 41.83 4 Figure S1: Representative 14C gel for cell-free IgG quantification Figure S1: Representative 14C gels used to calculate assembled IgG yields. These gels show the titration of sodium pyruvate at two potassium glutamate concentrations. This gel was used to calculate trastuzumab concentrations in Figure 4b in the main text. 5 Figure S2: Time course of trastuzumab expression using conventional and simplified protocols. Figure S2. Time course of trastuzumab expression in conventional and simplified protocols showing that the kinetics of expression are similar in the two systems. Expression was carried out on 100 mL scale in a DASGIP DASbox mini-bioreactor. Reactions were performed in triplicate. Protein expression was quantified by purifying 0.6 mL of each cell free reaction using a 40 µL protein A phytips (Phynexus) on a BioMek FX (Beckman Coulter) liquid handler. The final recovery yields were about 350 mg/L and protein recovery was estimated to be 50%. 6