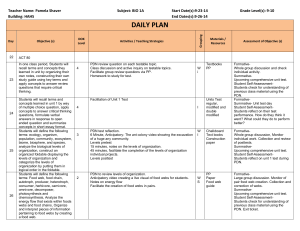
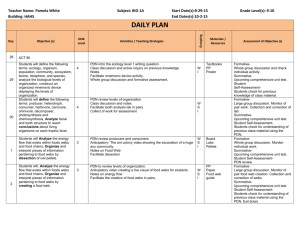
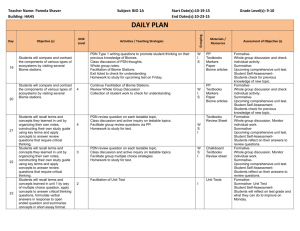
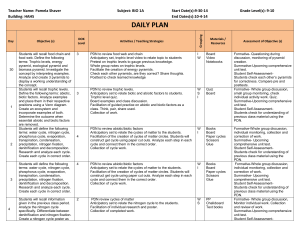
Protocolized Treatment Options for high severity localized scleroderma
advertisement

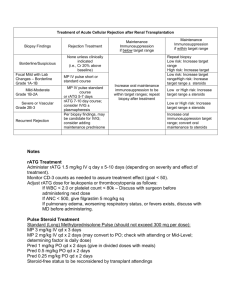
Development of Evidence Based “Best Standard Treatments” in Pediatric Rheumatology Carol A. Wallace MD Professor of Pediatrics University of Washington School of Medicine Seattle Children’s Hospital and Research Institute Immediate Past Chair of CARRA Why do we want “Best Standard Treatments”? • Standardized care results in decreased variation in patient care, which has been shown to result in improved quality and outcomes • Enable comparative effectiveness research to identify the safest, most effective and most cost effective treatments • Prevent complications by use of safest possible treatments • Disseminated widely to improve overall standards of patient care nationwide The National Childhood Cancer Mortality Rate and the Pediatric Cooperative Groups 8 Mortality per 100,000, AgeAdjusted Annual USA Cancer Mortality Rate Children <15 Years 6 4 IRSG ( ) NWTSG 2 SWOG Pediatric Division POG CALGB Pediatric Division C O G CCG 1950 1960 1970 1980 1990 2000 2005 How do we develop evidence based Best Standard Treatments? • Analyze data from clinical trials • Analyze data from large prospective cohorts followed from initial presentation with detailed information on treatment and outcomes We have neither available data sources… In the absence of data, one approach is to start with development of “Consensus Treatment Plans” • Consensus formed; with broad input • Reasonable, common and practical • Once developed, these can facilitate the development of evidence based Best Standard Care by: – Decreasing treatment variation – Enabling analysis of prospectively collected data of presenting features, treatments and outcomes In response to NIAMS RFA 05-AR-102 Comparative Effectiveness Research in Pediatric Rheumatic Diseases: Leveraging CARRA for Improved Child Health “CARRA- ” Childhood Arthritis and Rheumatology Research Alliance CARRA is: A research net work of North American Pediatric Rheumatologists and Health Care Professionals dedicated to improving the treatment and health related outcomes of children with rheumatic diseases through clinical research. CARRA Site Map CARRA ORGANIZATIONAL STRUCTURE Elected Steering Committee JIA Research Committee Chairs of the Disease Committees Small and Large Center Reps, Finance Chair, Past Chair Chair, Vice Chair, SLE Research Committee JDM Research Committee Pain Research Committee Small Center Rep Large Center Rep 304 members (71 trainees) Vasculitis/Scleroderma Research Committee Translational Research and Technology Committee Finance Committee 92 SITES www.carragroup.org What makes CARRA a Network? Member Participation Disease Specific Committee work Conduct of Research Education, training and mentoring Annual Meeting Collaborations with other organizations A Research Network Can… Improve the standard of care through clinical studies Promote a “culture” of research at Pediatric Rheumatology sites Develop Best Standard Treatments for better patient outcomes Specific Aims CARRA• To develop multiple standardized treatment plans for use in new onset patients with: JIA, SLE, JDM and localized scleroderma – Use of consensus methodology – Specify of outcome measures, data collection details and time points • Develop mechanisms to enable rapid dissemination and use of the consensus developed treatment plans • Develop analysis plans for the data collected to determine the clinical efficacy and safety of patients treated with these plans Overall Concept of CARRA• When a new patient presents, a physician would choose to use one of the standardized treatment plans that most fits what they would normally do. • Data would be collected on the patients treated with these standardized treatments • Data would be analyzed to determine the most efficacious treatment with the least side effects • Thru this process, we would create evidence based “Best Standard Treatment” CARRACarol Wallace (contact PI) Norm Ilowite (PI) JDM Module Linear Scl Module Ann Reed - Leader Adam Huber Co-leader Rob Fuhlbrigge-Leader JIA Module SLE Module Yuki Kimura- Leader Emily Von Scheven Leader Esi Morgan DeWitt Co-leader Hermine Brunner Co-leader Suzanne Li Co-Leader CARRA- Advisory Board Overall Grant Consultant Chair- Edward Giannini Dan Solomon, Brian Feldman, Pamela Weiss, Peter Boehm, Vincent Del Gaizo Carol Wallace (contact PI) Norm Ilowite (PI) Dan Solomon JDM Module Linear Scl Module Ann Reed -Leader Adam Huber Co-leader Rob Fuhlbrigge-Leader JIA Module SLE Module Yuki Kimura-Leader Emily Von Scheven Leader Esi Morgan DeWitt Co-leader Hermine Brunner Co-leader Suzanne Li Co-Leader CARRA- Advisory Board Overall Grant Consultant Chair- Edward Giannini Dan Solomon, Brian Feldman, Pamela Weiss, Peter Boehm, Vincent Del Gaizo Carol Wallace (contact PI) Norm Ilowite (PI) Dan Solomon JDM Module Linear Scl Module Ann Reed - Leader Adam Huber Co-leader Lay Members Rob Fuhlbrigge -Leader JIA Module Yuki Kimura-Leader Esi Morgan DeWitt Co-leader Lay Members SLE Module Emily Von Scheven Leader Hermine Brunner Co-leader Lay Members Suzanne Li Co-Leader Lay Members Consensus Methodology Choices • Convene a panel of experts • NIH model: – Public discussion of questions and evidence before an expert panel, followed by private unstructured deliberations of the panel • Delphi Questionnaire Technique • Nominal Group Technique • Combination of the Delphi Questionnaires and Nominal Group Technique Development process for Consensus Treatment Plans ***** Workgroup***** Development process for Consensus Treatment Plans Initial CARRA wide surveys JIA: new onset systemic JIA SLE: new onset nephritis JDM: new onset moderate to severe disease Localized scleroderma: new onset ***** Workgroup***** Development process for Consensus Treatment Plans CARRA wide survey Refinement, finalization of plans Consensus Meeting CARRA wide, case-based treatment survey Review of literature ***** Workgroup***** Treatment of Linear Scleroderma: Survey of Current Practice Case 1: 4yo F with 2 mo history of linear scleroderma of lower leg. Lesion crosses ankle and initially had erythematous- violaceous border. Now warm with SQ tissue loss. Labs show ESR Treatment of Linear Scleroderma: Survey of Current Practice Case 1: 4yo F with 2 mo history of linear scleroderma of lower leg. Lesion crosses ankle and nitially had erythematous- violaceous border. 4% use MTX only MTX only CS + MTX Now warm with SQ tissue loss. Labs show ESR 96% use methotrexate (MTX) + corticosteroid (CS) This looks pretty good. But what about treatment specifics? Li et al. J Rheum 2010;37: 175-81 What type of CS were chosen? Case 1: 4 yo F with linear scleroderma of leg 4% do not use CS no CS Oral CS only IV CS only IV + Oral CS 27% use IV + Oral CS 30% use oral CS 39% use IV CS How about specific CS doses and dosing regimens? CS use in LS: Dosing regimens and duration 3 main oral CS regimens: -1 mg/kg/d (28) -2 mg/kg/d (18) -0.5 mg/kg/d (11) 4 IV CS regimens: -3d/mo (33) -1/wk (19) -3d/wk (19) -1/mo (5) Pred 1mg/kg/d x 1 mo: 9 PR IV 3d/mo x 3 mo: 27 PR Pred 0.75x6 Pred 0.5 x1 Pred 0.5 x2 Pred 0.5 x6 Pred 0.5 xNS Pred 1 x1 Pred 1 x2 Pred 1 x 2.5 Pred 1 x3 Pred 1 x6 Pred 2 x0.5 Pred 2 x1 Pred 2 x2 Pred 2 x3 Pred 2 x6 Pred 2 xNS IV 1/wk x1 IV 1/wk x2 IV 1/wk xNS IV 1/mo x1 IV 1/mo x6 IV 1/mo x7 IV 1/mo x18 IV 3/mo x1 IV 3/mo x3 IV 3/mo x24 IV 3/mo+add IVMP IV 3/mo xNS IV 3/wk x0.25 IV 3/wk x0.5 +add IVMP IV 3/wk x3 IV 3/wk x NS IV 1/mx6 + pred2/kg x 12 IV 1/m x1 + pred1/kg x1 IV 1/wk x1 +pred 0.5 x1 IV 1/wk x3 +pred0.5x3 IV 1/wkx1 +pred 1/kgx1 IV 1/wk xNS +pred 1/kg x3 IV 1/wkx1 +pred 2/kg x1 IV 1/wkx1 +pred 0.5x1 IV 1/wkx2 +Pred 2/kgQOD x1 IV 1/wk x2 +pred 2/kg x1 IV 3/mo x1 then pred IV 3/mox1 +pred2/kg QOD x1 IV 3/mox3 then pred IV 3/mox1+pred 1/kgx1 IV 3/mo x3 +pred 1/kgx1 IV 3/mox6 +pred 1/kgx1 IV 3/wkX0.25 then pred IV 3/wkX0.25 +pred0.5/kg x2 IV 3/wk x0.25 +pred 0.5x1 IV 3/wk X0.25 +pred2/kg x2 IV 3/wk X0.25 +pred 1/kg x3 IV 3/wk xNS =Pred 1/kg x1 IV 3/wk x0.5 +pred 1/kg x3 IV 3/wk x0.25 +pred 2/kg x1 IV 3/wk x0.5 +pred 2/kg x1 IV 3/wk x0.5 +pred 2/kg x3 IV 3/wk x1 +pred 2/kg x1 IV 3/wk x0.25 +pred 1/kgx 1 MTX Treatment Regimens in LS 13 different initial doses 27 different initial doses and routes 0.3 pox15 0.3, 0.6 sq/po x 25 0.5 sq x7 0.5 sq x12 0.5 sq x 13 0.5,1 sq x14 0.5, 0.3 sq x21 0.5 sq x24 0.5 sq/po x36 0.5 NS xNS 0.6sq x15 0.6 sq/po, po x NS 0.75, 1 sq, 0.5po x 24 0.75 sq x 24 0.75 po x NS 0.875 sq/po, 0.75 sq, 1 po x 14 1 sq x 3 1 sq, 20 sq x13 1 sq x 15 1 sq/po, 15 sq/10po x 18 1 po x 24 1 sq, 0.3po x 24 1 sq x 27 1 sq/po x 30 1 sq x 36 1 sq x 48 1 po, 1 taper x 18 1 sq/po, taper x>12 1 sq x>12 1 sq x 36+ 1 sq, 15 po x 24_ 10 po x 15 10 sq/po, 15 sq/po x30 12 sq/po x 24 12.5 sq/po,1sq,, 10 po x12 15 sq x12 15 sq x15 15 sq x 24 15 sq/po x30 15 sq/po x 42 15 sq/po x 48 15 x sq x NS 20 sq x 15 0.3 po x24 0.5 sq x6 0.5 sq x 9 0.5, 1 po x 13 0.5, 0.75 sq x 13 0.5 sq x18 0.5 po x24 0.5, 1 sq x26 0.5 sq x12+ 0.6 po x12 0.6 sq xNS 0.75 po x12 0.75 po x24 0.75 sq x30 0.8 sq x NS 0.875 sq/po x 24 1 sq x12 1 sq/po, 1 sq x15 1 sq, 0.5 sq x 18 1 sq x 18 1 sq x 24 1 po x 27 1 sq, 0.75sq x30 1 sq x30 1 sq x 42 1 sq/po x 48 1 sq/10 sq x24+ 1 sq, taper x>10 1 sq, 0.5 sq x 12+ 1 sq x NS 10 sq x13 10 sq/po, 15 po x 26 10 po x NS 12 po x NS 15 po x 12 15 sq/po x 12 15 po x 24 15 po, 10 po x 26 15 sq x 36 15 sq x 48 15 po x NS 17.5 sq x 24 20 po x 21 Development process for Consensus Treatment Plans CARRA wide survey Refinement, finalization of plans Consensus Meetings at CARRA Annual meeting 2010 Case-based survey CARRA wide survey Review of literature ***** Workgroup***** Consensus methodologies facilitated and promoted the highest quality team work However, this is REALLY hard and time consuming! CARRA- CARRAGuiding Principles • Evidence-based when possible – Considered the best available literature • Compatible with the current practice – Assessed by the surveys • Consensus agreement – Delphi Questionnaires – Nominal group technique Development of Consensus Standardized Treatments • Distill what we do- using collective judgment and consensus processes • Forced us to: – Think critically about what we do – Use facts when present, not beliefs or “this is how I always do this” – Emphasis on decreased variation Development of Consensus Standardized Treatments • Not rigorous protocols- but rather menus of standardized treatments • Not perfect or exactly what each physician might do, but what he/she can live with • The goal is to reduce variation so we can treat in a standardized fashion, collect data and compare patient outcomes Common Module Work • Define the patient characteristics and exclusions for use of the treatment plans • What to do if better, worse or adverse event • Essential data to be collected • Essential labs to be collected • Key data collection time points Challenges • Steroids – How to standardize dosages and routes – Standardization of steroid taper rate • JIA: – Patient characteristics: how early to allow treatment may need to treat before fulfilling ILAR criteria for JIA – Include a new medication not yet approved? • SLE: Induction and maintenance, or just induction? • Linear scleroderma: – Lack of outcome measures – Lack of definitions for improvement, worsening SLE Breakout sessions Topic Group Coordination M= moderator N= note taker 1 Outcome measures/response variables M: Hermine Brunner N: Joyce Hsu 2 Cyclophosphamide plan M: Emily von Scheven N: Eyal Muscal 3 Mycophenolate plan M: Anne Eberhard N: Marisa Klein-Gitelman 4 Steroid regimens – doses and tapering M: Lynn Punaro N: Linda Wagner-Weiner 5 QOL/patient perspectives/ AEs M: Stacey Ardoin N: Nandini Moorthy, Jenny Palter Localized Scleroderma Consensus Treatment Plans Regimen A B MTX alone MTX + Intravenous steroid C MTX + Oral prednisone MTX weekly 1mg/kg SQ dose (max 25 mg) 1mg/kg SQ (max 25 mg) 1mg/kg SQ (max 25 mg) Steroid dose, Duration None 30 mg/kg/dose (max 1gm) 2 mg/kg/d (max 60 mg) divided EITHER: 3 consecutive bid daily doses/mo, Duration: at least 2 weeks OR: 1 dose/week Duration: 3 months Steroid taper targets None None Dose reduced to: 1 mg/kg/d (max 30 mg) by end of wk 8 0.5 mg/kg/d (max 15 mg) by end of wk 16 0.25 mg/kg/d (max 7.5 mg) by end of wk 24 Off CS by end of week 48 JIA Module • Developed 4 treatment plans: – Steroid only – Methotrexate – Anakinra – Tocilizumab • Possible steroid plans : – IV 30/kg/day X 3 days – Oral • 1 mg/kg/day – taper over 1, 3 or 6 months • 2 mg/kg/day – taper over 1, 3 or 6 months Plan B: Methotrexate Methotrexate (MTX) 0.5mg/kg PO or SQ weekly Optional corticosteroids : PDN 1mg/kg (60mg max); IV MP pulse 30mg/kg X 3 d ASSESS AT 1-2 WEEKS Improved Taper PDN (if on) (choose rapid, fast, slow) Unchanged Worsened Continue same dose PDN If not on oral PDN, add at 1mg/kg; Optional: Add or repeat IV MP (can be weekly) If on PDN, increase to 2mg/kg; Add/Repeat IV MP (can be weekly) ASSESS AT 1 MONTH If Worse If Improved If Unchanged Continue same dose MTX Increase MTX to 1mg/kg (30mg max) SQ weekly ; Continue same dose PDN , reassess monthly Start/Continue PDN taper, reassess monthly Increase MTX to 1mg/kg SQ weekly; Increase (or continue) PDN to 2mg/kg (max 100mg); repeat IV MP, reassess monthly ASSESS AT 3 MONTHS If Improved *If on PO PDN, taper PDN Continue same dose MTX, reassess monthly If Unchanged , Worse, or on >50% steroid dose Continue same dose PDN and MTX , Plus Add additional therapy, choose and follow either: anakinra plan or tocilizumab plan Systemic JIA data collection Category Items History demographics, date symptom onset, comorbidities, medications, recent rash or serositis, suspected MAS Patient and Physician assessments pain HRQOL physical function parent/patient global assessment provider global assessment Physical Exam Ht, Wt, BMI, active joint count, hepatomegaly, lymphadenopathy, rash, serositis, splenomegaly Labs CBC c-reactive protein, ESR, ferritin albumin, LDH Treatment-related factors Medications SAE or IME if discontinuation then provide reason Development process for Consensus Treatment Plans CARRA wide survey Consensus Meeting Case-based CARRA wide survey Review of literature ***** Workgroup***** Refinement, finalization of plans; CARRA 2011 meeting Consensus Treatment Plans developed for new onset: • Systemic JIA – – – – Steroids MTX +/- steroids Anakinra +/steroids Tocilizumab +/steroids • Juvenile Dermatomyositis – Pred + MTX – Pred + MTX + IV methylpred – Pred + MTX + IV methylpred + IVIG • Renal disease in SLE – Cell cept induction – IV cytoxan induction • Linear Scleroderma – MTX – MTX +Oral steroid – MTX + IV methyl pred •All Modules have submitted the Treatment Plans and their development for publication Next Steps • Parallel to this effort has been the development of a large foundational pediatric rheumatology registry: CARRAnet – 60 participating pediatric rheumatology sites – 6,000 patients enrolled • Plan is that new onset patients treated with one of the Consensus Treatment Plans will have specific data collected using the CARRAnet platform Consensus Treatment Plans CARRA website Publication progress: JDM – AC&R SLE – AC&R Systemic JIA – revision AC&R Localized scleroderma – revision AC&R Implementation of Consensus Treatment Plans Protocol for the CARRA Registry and Consent form allows for data collection that will cover the basic registry and additional sub-studies. Actual additional data collection will occur at sites participating in the pilot studies. All CARRA members are encouraged to use the CTP on new patients, regardless of participation in the pilot studies Consensus Treatment Plans: Pilot Studies JDM: Friends of CARRA and CUREJM 10 sites/ 40 patients Systemic JIA: Arthritis Foundation 15 sites/ 30 patients SLE: Lupus Foundation of America 15 sites/ 40 patients Localized Scleroderma: Arthritis Foundation 11 sites/ 50 patients Future Steps • Analysis of the data collected through the CARRAnet platform will allow for comparisons between treatments for determination of effectiveness, outcomes and side effects • Evidence based Best Standard Treatments can then be identified and widely disseminated • Over time these can be improved upon in an iterative fashion to improve the outcomes of children with rheumatic diseases • Development of standardized treatment plans for additional phases/categories of disease are underway Plan A: Steroid Prednisone (PDN) 1mg/kg (max 60mg) daily Optional IV MP pulse 30mg/kg (max 1g) daily for 3 days ASSESS AT 1-2 WEEKS Improved Unchanged Worsened Taper PDN Continue same dose PDN (choose rapid, fast, slow) Add or repeat IV MP Increase PDN to 2mg/kg (max 100mg); Repeat IV MP ASSESS AT 1 MONTH If Unchanged or Worse If Improved Continue (or initiate) PDN taper Increase (or continue) PDN to 2mg/kg (max 100mg); repeat IV MP (can be weekly), reassess monthly ASSESS AT 3 MONTHS Patient on <50% starting steroid dose Patient on >50% starting steroid dose Continue to taper PDN, reassess monthly Continue same dose PDN , Plus If improved, continue PDN taper Add additional therapy, choose and follow either : If unchanged or worsened, add additional therapy (plan B, C, D) methotrexate, anakinra, tocilizumab plan Plan C: Anakinra Anakinra (ANK) 2mg/kg (max 100mg) SQ daily Optional corticosteroids : PDN 1mg/kg (60mg max) with IV MP pulse 30mg/kg/d X 3 ASSESS AT 1 -2 WEEKS Improved Unchanged Continue same dose ANK If on PDN, taper (choose rapid, fast, slow) Continue same dose ANK If on PDN, continue same dose Optional: Add or repeat IV MP Worsened Increase ANK to 4mg/kg (200mgmax) SQ daily; If on PDN, continue same dose Optional: Add or repeat IV MP ASSESS AT 1 MONTH If Unchanged If Improved Continue same dose ANK Start/Continue PDN taper, reassess monthly If Improved If Worse Increase ANK to 4mg/kg (200mg max) SQ daily; Continue same dose PDN reassess monthly Continue same dose ANK If not on oral PDN, add or increase PDN to 2mg/kg; Add/Repeat IVMP; reassess monthly ASSESS AT 3 MONTHS *If on PO PDN, taper PDN If Unchanged , Worse, or on >50% steroid dose Continue same dose PDN Continue same dose ANK Plus either reassess monthly add MTX, or switch to tocilizumab plan Plan D: Tocilizumab Tocilizumab (TOC) 8mg/kg (if >30 kg) or 12 mg/kg (if <30 kg) IV every two weeks Optional corticosteroids: PDN 1mg/kg (60mg max) with IV MP pulse 30mg/kg/d X 3 ASSESS AT 2 WEEKS Improved Unchanged Worsened If on PDN, taper If on PDN, continue same dose (choose rapid, fast, slow) Optional: Add/Repeat IV MP If not on oral PDN, add at 1mg/kg If on PDN, increase to 2mg/kg; Optional: Add/Repeat IV MP ASSESS AT 1 MONTH If Improved If Unchanged or Worse Continue same dose TOC Continue same dose TOC; If not on oral PDN, add; If on PDN increase (or continue) to 2mg/kg (max 100mg); repeat IV MP , reassess monthly Start/Continue PDN taper, reassess monthly If Improved ASSESS AT 3 MONTHS *If on PO PDN, taper PDN If Unchanged , Worse, or on >50% steroid dose Continue same dose PDN /IV MP pulses Continue same dose TOC Plus either reassess monthly add MTX or switch to anakinra plan