Density Problems
advertisement

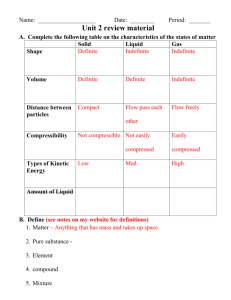
Unit 0-1 Station Review In order to perform at the highest possible level, you will need to do the following actions: 1. Go through your learning log sheet and note the learning goals that you need the most assistance with. 2. Complete the Station Review Problem that follows the essential learning targets. 3. Complete flash cards for terms that you still need help on that are in your glossary. See each learning goal for the terms that you need to know. 4. See Mr. Astor for help Learning Goal 1 2 3 4 5 6 7 8 9 10 SWBAT Distinguish between a physical change and a chemical change (chemical and physical changes can be exothermic or endothermic). Use a simple particle model to differentiate among properties of the 3 states of matter: solids, liquids and gases. Use particle diagrams and models to differentiate among elements, compounds, pure substances and mixtures Distinguish a mixture (homogenous or heterogonous, components can be physically separated due to differing properties e.g. density, boiling point etc., proportions of components can vary, each component retains its original properties) from a pure substance (has constant composition, constant properties throughout the sample). Describe the process and use of filtration, distillation, and chromatography in the separation of a mixture. Describe the units of intrinsic physical properties such as density, and detail how this can represented numerically, physically, atomically, graphically, and through calculation. Describe the general structure of the four important macromolecules, their building blocks, and what role each plays in human nutrition and food preparation. In particular, explain what proteins are made of, why the protein’s structure matters, and what happens when it is denatured. Define potential, kinetic, and thermal energy as well as their applications in the cooking process. Describe the difference between heat and temperature, as well as distinguish and interconvert between the 3 units of temperature measurement. Explain the meaning of endothermic and exothermic in terms of enthalpy change, and discuss how changes in enthalpy relate to changes in temperature and to changes in phase. Detail how to calculate the quantity of heat required to take a substance from one end of the x-axis on a heating curve to the other end of the x-axis. Generate and analyze data from scientific experiments and represent it graphically or in table form, as well as distinguish between a quantity and a unit. Demonstrate how to interconvert between values using dimensional analysis. Current Score on this Standard Place a for 83 or higher Learning Goal 1: Distinguish between a physical change and a chemical change (chemical and physical changes can be exothermic or endothermic). Lesson 1.2 – Physical and Chemical Changes Confused? Go to these notes! 1. Which particle diagram represents a physical change only? 2. Identify each of the following as either a physical change or a chemical change. Place a check in the correct column of the table. The first has been done for you. 3. Identify the following as physical (P) or chemical (C) changes _____ Road salt, sodium chloride, is commonly used to de-ice roads during the winter. When road salt dissolves in the water on the road, it reduces the temperature at which the water would freeze. This helps prevent ice from forming on the roads. _____Cars have been made from steel, which is mostly iron, since their introduction into society in the early 20th century. When the iron interacts with oxygen, it forms rust. This problem is accelerated by the wet, salty roads in many cold, winter climates. _____Copper has been used by humans for about 10,000 years. Due to its excellent flexibility and great ability to conduct electricity, copper is used for electrical wires as well as in pipes for plumbing. Learning Goal 2: Use a simple particle model to differentiate among properties of solids, liquids and gases. Lesson 1.1 – States of Matter and Physical Composition Confused? Go to these notes! 1. Draw particle diagrams for solids, liquids, and gases. SOLID LIQUID 2. Circle the diagram that best represents a solid in a closed container. GAS 3. Which sample is most likely to take the shape of and occupy the total volume of its container? (A) CO2 (g) (B) CO2 (l) (C) CO2 (aq) (D) CO2 (s) 4. Which substance has a definite shape and a definite volume at STP? (A) H2O (l) (B) Cl2 (g) (C) CCl4 (l) (D) AlCl3 (s) 5. Which state(s) of matter are compressible? Which state(s) of matter are not compressible? Explain. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ Learning Goal 3: Use particle diagrams and models to differentiate among elements, compounds, pure substances and mixtures 1.2.Lab 2 – Aluminum Foil Transformer Lab Confused? Go to these notes! 1. In the space provided below, draw the particle models for the following substances: Mixture Solid 2. Which Gas diagram or diagrams represent a pure substance of diatomic element A? (A) X, only 3. Given: Element (B) Y, only (C) Z, only (D) X and Z which diagram represents a mixture? 4. Draw in the box below a particle diagram to represent a heterogeneous mixture of diatomic oxygen and hydrogen peroxide, H2O2. Draw at least three of each type of molecule. Use the key provided. Hydrogen = Oxygen = Learning Goal 4: Distinguish a mixture (homogenous or heterogonous, components can be physically separated due to differing properties e.g. density, boiling point etc., proportions of components can vary, each component retains its original properties) from a pure substance (has constant composition, constant properties throughout the sample). Lesson 1.1 – States of Matter and Chemical Composition Confused? Go to these notes! 1. Identify one or more of the following as a heterogenous mixture(s): (1) Ocean Water (3) Milk (2) Sand and Water (4) Heavy Cream 2. Which of these contains only one substance? (1) distilled water (2) sugar water (3) saltwater (4) rainwater 3. Which particle diagram below represents a heterogeneous mixture? A. B. C. D. 4. List out the changes in substances you observed in the Aluminum Foil Transformer lab and justify why the reactants or products are elements, compounds, heterogenous mixtures, or homogeneous mixtures: __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ Learning Goal 5: Describe the process and use of filtration, distillation, and chromatography in the separation of a mixture. Lesson 1.3 – Data Analysis and Digestion Chemistry Confused? Go to these notes! 1. Which two physical properties allow a mixture to be separated by chromatography? (1) Hardness and boiling point (2) Density and specific heat capacity (3) Malleability and thermal conductivity (4) Solubility and molecular polarity 2. At room temperature, a mixture of sand and water can be separated by: (1) Ionization (3) Filtration (2) Combustion (4) Sublimation 3. Which process would most effectively separate two liquids with different molecular polarities? (1) Filtration (3) Distillation (2) Fermentation (4) Conductivity 4. A bottle of rubbing alcohol contains both 2 – propanol and water. These liquids can be seperated by the process of distillation because 2 – propanol and water (1) have combined chemically and retain their different boiling points (2) have combined chemically and have the same boiling point (3) have comined physically and retain their different boiling points (4) have combined physically and have the same boiling point 5. When a mixture of water, sand, and salt is filtered, what passes through the filter paper? (1) Water, only (2) Water and sand, only (3) Water and salt, only (4) Water, sand, and salt 6. At equilibrium, nitrogen, hydrogen, and ammonia gases form a mixture in a sealed container. The data table gives some characteristics of these substances. Describe how to separate ammonia from hydrogen and nitrogen using filtration. __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ Learning Goal 6: Describe the units of intrinsic physical properties such as density, and detail how this can represented numerically, physically, atomically, graphically, and through calculation. Lesson 1.0 – Matter and Density of Different Dairies Lab Confused? Go to these notes! 1. What is the difference between mass of an object and its volume? 2. If the box at left contains atoms of aluminum in the liquid phase, represent the same atoms in the solid phase in the box at right. 3. How would you represent the atoms of aluminum in the gaseous phase? 4. If the box at left contains atoms of iron in a cast iron skillet, represent the atomic structure of the iron skillet after strong heating in the box at right. Density Problems 5. Mercury has a density of 13.6 g/mL. What is the volume occupied by 112.0 grams of mercury? 6. A cube of gold-colored metal with a volume of 54 cm3 has a mass of 980 g. The density of gold is 19.3 g/cm3. Is this sample of metal pure gold? Why or why not? Learning Goal 6: Describe the units of intrinsic physical properties such as density, and detail how this can represented numerically, physically, atomically, graphically, and through calculation. 1.0.Lab 1.Density of Different Dairies Lab Confused? Go to these notes! A student graphed the following data: 1. Based on this graph, how does metal A differ from metal B? 2. What is the density of metal A? Show all your work and include appropriate units. 3. What is the mass of 12.0 cm3 of metal A? Find this in two different ways. a. Mark on the above graph how you might determine this. b. Show your work on how you might also calculate this mathematically. A student graphed the following data: 1. Based on this graph, how does metal B differ from metal A? 2. What is the density of metal B? Show all your work and include appropriate units. 3. What is the mass of 9.0 cm3 of metal B? Find this in two different ways. a. Mark on the above graph how you might determine this. b. Show how you could calculate this mathematically. Learning Goal 7: Describe the general structure of the four important macromolecules, their building blocks, and what role each plays in human nutrition and food preparation. In particular, explain what proteins are made of, why the protein’s structure matters, and what happens when it is denatured. Lesson 1.3 and 1.4 – Protein Chemistry Confused? Go to these notes! 1. This molecule is the basic subunit of what larger biological molecule? 3. This molecule is the basic subunit of what larger biological molecule? 4. Briefly explain the different levels of protein structure, and what happens when heat is applied: ____________________________________________________________________________________________________________________________________ Learning Goal 8: Define potential, kinetic, and thermal energy as well as their applications in the cooking process. Describe the difference between heat and temperature, as well as distinguish and interconvert between the 3 units of temperature measurement. Lesson 1.5 and 1.6 – Thermodynamics of Cooking Part I and II Confused? Go to these notes! 1. A liquid has a critical temperature of 154.4 K; calculate the temperature in oF and oC 2. Detail how heat, temperature, and kinetic energy are all interrelated. 3. How does potential energy change as a substance changes phases? How does kinetic energy change as a substance changes phases? What is the reason for this? 4. Hot object (A) and cold object (B), each having the same number of particles, are brought into contact with each other. Which of the following is true? a. b. c. d. e. f. Energy flows only from object A to object B Energy flows only from object B to object A More energy flows from B A than A B More energy flows from A B than B A Equal flow of energy from A B and B A No exchange of energy between these objects occur Justify your choice. 5. Consider two objects A and B that are at the same temperature BUT Object A contains twice the number of particles in comparison to Object B. Which of the following is true? g. h. i. j. k. l. Energy flows only from object A to object B Energy flows only from object B to object A More energy flows from B A than A B More energy flows from A B than B A Equal flow of energy from A B and B A No exchange of energy between these objects occur Justify your choice. ____________________________________________________________________________________________________________________________________ Learning Goal 9: Explain the meaning of endothermic and exothermic in terms of enthalpy change, and discuss how changes in enthalpy relate to changes in temperature and to changes in phase. Detail how to calculate the quantity of heat required to take a substance from one end of the x-axis on a heating curve to the other end of the x-axis. Lesson 1.6 and 1.7 – Thermodynamics of Cooking Part II and III Confused? Go to these notes! 1. Define each of the quantities in the equation: q 2. What is the unit on each quantity? q______________ m______________ = m Cs ΔT ΔT______________ 3. Rearrange the equation given in Question 1 and solve for Cs What are the units for Cs? 4. A 175 g sample of water initially at 23.45 oC absorbs some heat. The final temperature of the sample after absorbing the heat is 26.85 oC. Calculate the amount of heat absorbed by the sample of water. (Specific Heat = 4.184 J/g x oC) 5.A piece of iron weighing 80.0 g initially at a temperature of 92.6 oC released the same amount of heat to the 175 g sample of water in Question 4. Assume the final temperature of the metal is the same as the final temperature of the water in question 4. What is the specific heat of iron? 6. For endothermic reactions at constant pressure a. ΔH < 0 b. ΔH > 0 c. ΔH = 0 d. ΔS > 0 e. ΔS < 0 7. A 57 gram block of metal at 92°C is dropped into an isolated flask containing approximately 45.0 grams of ice and 30.0 grams of water at 0°C. After the system had reached equilibrium it was determined that 9.5 grams of the ice had melted. What is the specific heat of the metal? (Heat of fusion of water = 333 J/g) a. 0.22 J/°C/g b. 0.32 J/°C/g c. 0.60 J/°C/g d. 0.79 J/°C/g e. 0.92 J/°C/g 8. Anna Litical and Noah Formula dry and mass out 25.8-gram of ice and place it into a coffee cup with 100.0 g of water at 35.4°C. They place a lid on the coffee cup and insert a thermometer. After several minutes, the ice has completely melted and the water temperature has lowered to 18.1°C. What is their experimental value for the specific heat of fusion of ice? The basis for the solution to this problem is the recognition that the quantity of energy lost by the water when cooling is equal to the quantity of energy required to melt the ice. In equation form, this could be stated as Qice = -Qcalorimeter (The negative sign indicates that the ice is gaining energy and the water in the calorimeter is losing energy.) Here the calorimeter (as in the Qcalorimeter term) is considered to be the water in the coffee cup. Since the mass of this water and its temperature change are known, the value of Qcalorimeter can be determined. 9. The Big Kahuna: Copy down the Iron Chef Wikispaces Challenge Problem and detail the process on how to solve each step here: ____________________________________________________________________________________________________________________________________ Learning Goal 10: Generate and analyze data from scientific experiments and represent it graphically or in table form, as well as distinguish between a quantity and a unit. Demonstrate how to interconvert between values using dimensional analysis. Lesson 0.3 – Sig Figs and Dimensional Analysis Confused? Go to these notes! 1. The diameter of the sun is 1,390,000 km. Demonstrate that in proper scientific notation: 2. What is the surface area of the sun in km2 and in mile2? 3. What is the volume of the sun in km3 and in mile3? 4. The mass of the sun is 1.989 x 1030 kg. What is the average density of the sun in kg/km3? 5. What is the average density of the sun in g/cm3? 6. Calculate the volume of a backpack in cm3, m3, and in3 whose dimensions are 22.86 cm x 38.0 cm x 76 cm. 7. Indicate the number of significant digits in each of the following measurements. a. 23.500 g b. 100.35 ml c. 1.004 x 10-7 m d. 0.00230 kg 8. Round of the following numbers to 3 significant figures. a. 0.0089346 kg b. 96515 mL c. 3.50492 m 9. Determine the result to the correct number of significant figures: (3.2 cm X 1.23 cm X 0.5cm) _ = (8.32 cm X 1.000 cm X 0.500 cm) 10. A typical soft drink container is 355 mL; determine he number of quarts of the soft drink container: ____________________________________________________________________________________________________________________________________