File
advertisement

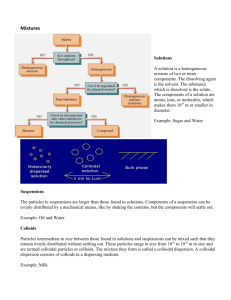
N. Vamsi Krishna Introduction: • Colloidal system are the ones in which one of three states (solid, liquid and gas) is finely dispersed in another. • Colloids were named first in the early 19th century by the Father of Physical Chemistry, Thomas Graham (1805-1869). In 1920's and 1930's, the importance of colloids to industrial processes and biochemistry changed everything making it a hot field. • A colloidal system consists of an internal phase (dispersion phase), which is the material of colloidal dimensions, and an external phase (dispersion medium) .Similar to the terms solute and solvent used for simple solutions. • As the particles of a colloid system become smaller and smaller, we go over imperceptibly from a two-phase colloid to a single-phase solution, and there is no definite boundary i.e. true solution. Table 1: Characteristics of Suspension, Colloid and Solution Properties Suspension Colloid Solution 1. Particle size >100nm 1-100nm <1nm 2. Separation 1)ordinary filtration 2) ultra filtration possible possible not possible possible not possible not possible Settles under gravity Settles on Centrifugation Does not settle opaque Generally clear clear Not possible Diffuses slowly Diffuses rapidly 3. Settling 4. Appearance 5. Diffusion 6. Brownian motion shows shows Not observable 7. Tyndall effect shows shows Not observable Classification of colloids: Colloids can be classified mainly by 3 ways; • Classification Based on the State of the Dispersed Phase and Dispersion Medium • Classification of Colloids Based on Type of Particles of the Dispersed Phase • Classification Based on the Nature of Interaction Between Dispersed Phase and Dispersion Medium Classification Based on the State of the Dispersed Phase and Dispersion Medium Table 2: Types of colloids Dispersion Medium Dispersed phase Type of colloid Example Gas Liquid Aerosol Fog, clouds Gas Solid Aerosol Smoke Liquid Gas Foam Whipped cream, soda water Liquid Liquid Emulsion Milk, hair cream Liquid Solid Sol Paints, cell fluids Solid Gas Foam Pumice, plastic foams Solid Liquid Gel Jelly, cheese Solid Solid Solid Sol Ruby glass Classification of Colloids Based on Type of Particles of the Dispersed Phase a) Multimolecular colloids usually have lyophobic character. Ex: gold and sulphur sols b) Macromolecular colloids resemble true solutions Ex: proteins, cellulose, starch and polymers such as polyethylene, nylon and polystyrene c) Associated colloids type of micelle it forms depends on the nature of solvent (hydrophilic or hydrophobic) Kraft Temperature (Tk ) Critical micelle concentration (CMC) Ex: soaps and synthetic detergents Surfactant Micelle Classification Based on the Nature of Interaction Between Dispersed Phase and Dispersion Medium Table 3: Distingustion between lyophilic and lyophobic colloids Property Lyophilic sols (suspensoid) Lyophobic sols (Emulsoid) Surface tension Lower than that of the medium Same as that of the medium Much higher than that of the medium Same as that of the medium Reversibility Reversible Irreversible Stability More stable Less stable Visibility Particles can’t be detected even under ultra microscope Particles can be detected under ultra microscope. Migration Particles may migrate in either direction or do not migrate in an electric field because do not carry any charge. Action of electrolyte Addition of smaller quantity of electrolyte has little effect Coagulation takes place Hydration Extensive hydration takes place No hydration Examples Gum, gelatin, starch, proteins, rubber etc. Metals like Ag and Au, hydroxides like Al(OH3), Fe(OH)3 metal sulphides like AS2S3 etc. Viscosity Particles migrate either towards cathode or anode in an electric field because they carry charge. Physical methods of preparing colloid: • By excessive cooling: used to prepare a colloidal solution of ice. • By exchange of solvent: Ex: S (alc) + H2O S (sol) • By change of physical state: Sols of substances like mercury and sulphur are prepared b) Dispersion method • Mechanical disintegration: Colloidal solutions of black ink, paints, varnishes, dyes etc. and food products like concentrated milk, food additives etc. Figure1: Pictorial view of colloidal mill • Peptization: The process of dispersing a precipitate in to a colloidal solution by adding small quantity of electrolyte is called peptization. Ex: Fe(OH)3 ↓+ FeCl3 [ Fe(OH)3Fe3+] (sol) + 3Cl¯ • Bredig’s arc method: used to prepare sols of platinum, silver, copper or gold. Traces of KOH are required to stabilize the colloidal solution. Figure 2: Bredig’s arc method Generally used methods to prepare other colloidal systems are; − Aerosol is formed by passing gas jet to a liquid spray. − Emulsions are usually prepared by vigorously shaking the two constituents together, often with the addition of an emulsifying agent . The phase in which emulsifier is more soluble forms the outer layer. lyophobic colloids . − Gels are often formed by cooling lyophilic sols that contain large linear molecules and have a much greater viscosity than the solvent. Elastic and Rigid gels. − Foams are formed when gas and liquid are mixed together in a container and shaken along with a foaming agent. Purification of colloidal solutions: • Colloidal solutions prepared by the above methods contain some soluble impurities and excess of electrolytes; these have to be removed to obtain pure sols. •Generally a) Dialysis, b) ultra filtration and c) ultra centrifugation are the techniques used • Ultra filtration: colloidal sols are filtered through ultra-filtrers. pore size of filter paper is decreased such that it will restrict the passage of colloidal particles. • Ultra-centrifugation: Centrifugation is carried out at very high speeds such that the colloidal particles settle down at the bottom of the tube and the impurities remain in the solution . • It is important to note that above methods to purify colloidal solution do not produce 100% solution. Figure 3: Electro-dialysis • used for purification of blood in case of kidney failure. Properties of colloids: a) Optical properties Figure 4: Size dependent change of colour in Au sol Figure 6: Schematic drawing of ultra microscope Opalescence in colloids is due to scattering of light by particles. This effect was studied by Tyndall and is generally known as tyndall effect. Tyndall observed that scattered beam to be polarized and intensity of the same to be dependent on position of observer, nature of system and wavelength of light used. b) Colligative Properties • The magnitudes of these properties for colloidal solutions are much smaller than those obtained for true solutions. They exhibit measurable osmotic pressures. This property is used for the determination of the average molecular masses of the colloids. c) Kinetic properties liquid undergoes continuous chaotic and random motions. This motion of the particles is called Brownian motion. This Brownian motion is found to decrease by increase in particle size or by increase in viscosity of medium. r = (3bυ/ 4π nd) ⅓ where, b = number of grams of substance per dm3 n = number of particles observed in view υ = volume dm3 d = density of dry substance c) Electrical properties Lyophobic sols carry charge, because of which they get repelled on approaching another particle avoiding their coagulation. i) Due to presence of electrolytes Ex: positive charge on ferric hydroxide sol prepared by hydrolysis of ferric chloride is due to adsorption of Fe3+ ions on surface. ii) dissociation of molecular electrolytes adsorbed on the surface of particles. Ex: H2S molecules get adsorbed on sulphides during precipitation. H2S undergoes dissociation and the hydrogen ions are lost. The particles become negatively charged due to (S2-) which are left on the colloidal particles. iii) by dissociation of surface molecules Ex: soaps, proteins Figure 7: Electrical double layer The influence of net charge decreases with distance and so the number of oppositely charged ions, equaling the number of ions of both charges prevailing electro neutrality. Properties of colloids: • Purification of water by alum (coagulation) • In rubber platting • In tanning • Artificial rains • Formation of deltas (coagulation) • Blood clot formation • Colloidal medicine: Argyrol and protargyrol are colloidal solution of silver and are used as eye lotions .Colloidal sulphur is used as disinfectant and colloidal gold, calcium and iron are used as tonics. A wide variety of medicines are emulsions. • Coating of Photographic plates • Sewage disposal • Metallurgy • Smoke precipitation (Coagulation) Figure 8: Schematic diagram of Cottrell smoke precipitator