03.Adsorption on the interphase of liquid
advertisement

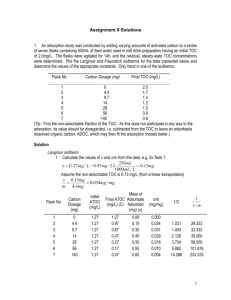
Lecture 3. Adsorption on the interphase of liquid-gas Prepared by PhD Falfushynska Halina • A mathematical equation, which describes the relationship between pressure (p) of the gaseous adsorbate and the extent of adsorption at any fixed temperature, is called adsorption isotherm. • The extent of adsorption is expressed as mass of the adsorbate adsorbed on one unit mass of the adsorbent. Adsorption Isotherms Data relating adsorbed concentration (g/g of bed weight) to equilibrium gas phase concentration (g/ml of stream) is given in terms of adsorption isotherms. Wads = f (P,T) Three common types of isotherms: Langmuir Freundlich BET Types of adsorption isoterms Favorable and Unfavorable Adsorption Langmuir Isotherm The earliest model of gas adsorption suggested by Langmuir (1916). The classical Langmuir model is limited to monolayer adsorption. It is assumed that gas molecules striking the surface have a given probability of adsorption. At equilibrium, equal numbers of molecules desorb and adsorb at any time. The probabilities are related to the strength of the interaction between the adsorbent surface and the adsorbate gas. Langmuir Adsorption Isotherm The surface of the adsorbent is uniform, that is, all the adsorption sites are equivalent. ---Adsorbed molecules do not interact. ---All adsorption occurs through the same mechanism. ---At the maximum adsorption, only a monolayer is formed. • The Langmuir equation is expressed here as: • where K = Langmuir equilibrium constant, c = aqueous concentration (or gaseous partial pressure), Γ = amount adsorbed, and Γmax = maximum amount adsorbed as c increases. • The equilibrium constant is actually given by : • The Langmuir equation can be fitted to data by linear regression and nonlinear regression methods. Langmuir Adsorption Isotherm θ= the number of sites of the surface which are covered with gaseous molecule, P= pressure K =is the equilibrium constant for distribution of adsorbate between the surface and the gas phase . Total number of sites, ST Langmuir Isotherm Rate of adsorption, ra ka P(1 ) Rate of desorption, rd kd At equilibrium, ka P ka P kd Wads CP Wmax 1 CP 1- air adsorbate where, Wads = the mass of gas adsorbed at pressure P; Wmax = the mass of gas which covers the entire adsorbing surface with a monolayer; P = the partial pressure of interest in the gas phase; = coverage; C = a constant for the gas/solid combination = ka/kd; ka = the adsorption rate coefficient; kd = the desorption rate coefficient. Langmuir Isotherm (cont’d) Some physisorption and most chemisoption processes follow this isotherm. It is the one with the best theoretical basis, which assumes that adsorption is limited to one monolayer on the surface. One can obtain the two constants by linearization of the isotherm: P 1 P Wads CWmax Wmax Wads CP Wmax 1 CP 1 S = 1/Wmax P/Wads C J = 1/CWmax 0 P P Freundlich Isotherm The Fruendlich isotherm model is valid for heterogeneous surfaces, monolayer coverage. Common for most adsorption work since it fits almost all data. It is empirical in nature, although some theoretical foundations do exit. Freundlich Isotherm The expression: Wads = KF P 1/n (KF and n are experimentally determined parameters) n>1 n=1 Wads n<1 P When n = 1, the reaction is linear and called “partitioning”. When n > 1, the reaction is said to be “favorable” as the incremental change in amount sorbed decreases with increasing concentrations. While n < 1 is called “unfavorable” because the reverse is true. Most natural adsorbents exhibit either linear or favorable adsorption. The Langmuir and Fruendlich models for n < 1 are concave downwards, so both models can be calibrated to similar data.. Freundlich Isotherm Parameters Available for a wide variety of organic vapors on various activated carbon types Wads = KF P 1/n Example The Freundlich isotherm can be accepted to represent equilibrium concentrations of phenol in the g/L concentration range and x/m represents g phenol/mg C. a) If the limiting concentration of phenol is set at 0.2g/L and the source contains 30g/L, calculate the required dosage of powdered activated carbon. b) If instead of a single dosage, the carbon was dosed twice, first to achieve a concentration of 3g/L and then in a second tank to the final requirement, how much carbon will be required? c) How could we minimize carbon requirements over two dosages? d) Qualitatively consider the effect of even more tanks and possibilities of reusing the carbon from tank to tank. The isotherm may be extrapolated. Freundlich isotherm for Problem a) According to the graph, when c = 0.2 g/L, x/m = 0.019, assuming 1L phenol solution: (30g / L 0.2g / L) 1L 29.8g 0.019gphenol / mgC m 1568mgC m(mg ) 0.019gphenol / mgC b) According to the graph, when c = 3 g/L, x/m = 0.089, assuming 1L phenol solution: (30g / L 3g / L) 1L 27 g 0.019gphenol / mgC m 303.4mgC m(mg ) 0.089gphenol / mgC According to the graph, when c = 0.2 g/L, x/m = 0.019, assuming 1L phenol solution: (3g / L 0.2g / L) 1L 2.8g 0.019gphenol / mgC m 147.4mgC m(mg ) 0.019gphenol / mgC So, the total carbon required is: 303.4 mgC+147.4 mgC = 450.8 mgC c) In order to minimize the carbon usage over two dosages, a concentration of the effluent of phenol from the first activated carbon dosage must be determined. If this concentration is too close to the initial phenol concentration, the dosage used in the second step will be decreased while that used in the first step will be increased, and vice versa. More elegantly, write and equation of C as a function of PAC dose, differentiate and solve when = 0. PAC dosages of about 160 mg/L each will represent the lowest dosage for a two-step process. d) The total dosage needed will decrease with an increase of tanks. If the carbon from the last step is recycled towards the first tank, countercurrent to the water flow, the adsorption capacity of carbon can be maximized and minimize the carbon requirement even further. You can see that the PAC from the last step above would have much adsorption capacity left when reused in the first tank. Brunauer-Emmett-Teller (BET) Isotherm Brunauer, Emmett and Teller (BET) developed several models for gas adsorption on solids which have become the effective standard for surface area measurements. BET isotherm is valid for multiple layers on homogeneous surfaces. The assumptions used to derive the BET isotherm are • 1. Gaseous molecules behave ideally • 2. Multiple nitrogen molecules can be adsorbed to each site • 3. Each adsorbed molecule provides a site for the adsorption of the molecule in the layer above it • 4. All sites on the surface are equivalent • 5. No adsorbate - adsorbate interactions • 6. An adsorbed molecule is immobile • 7. Nitrogen in the second and higher layers are assumed to be liquid like