sia5925-sup-0001-Supplementary
advertisement
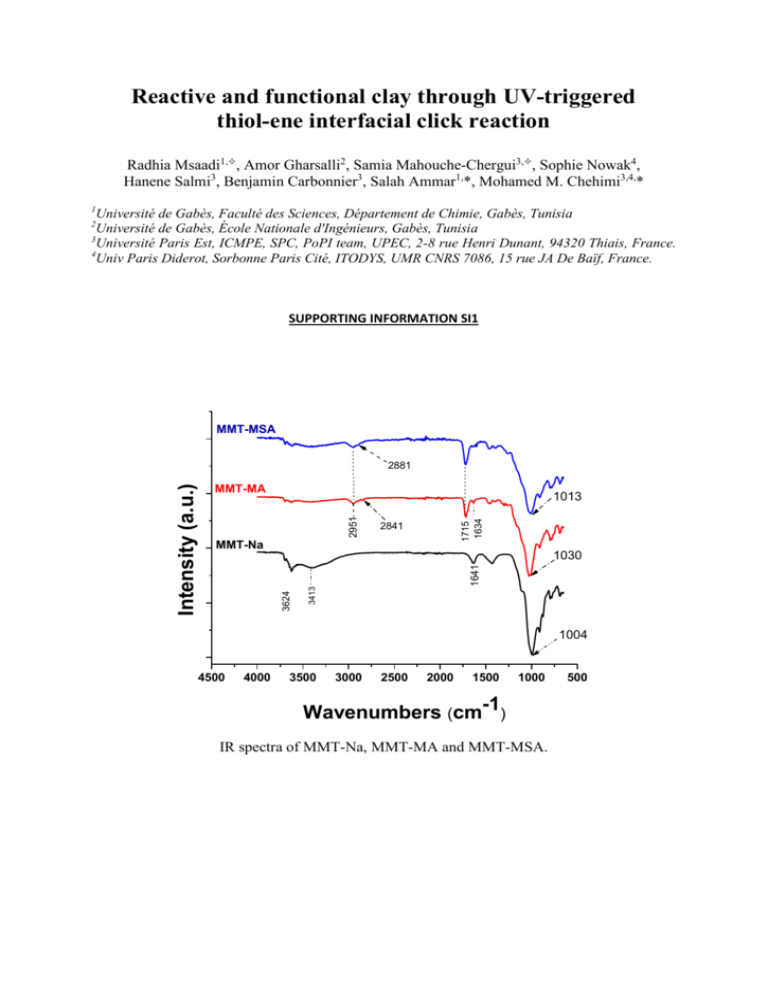
Reactive and functional clay through UV-triggered thiol-ene interfacial click reaction Radhia Msaadi1,, Amor Gharsalli2, Samia Mahouche-Chergui3,, Sophie Nowak4, Hanene Salmi3, Benjamin Carbonnier3, Salah Ammar1,*, Mohamed M. Chehimi3,4,* 1 Université de Gabès, Faculté des Sciences, Département de Chimie, Gabès, Tunisia Université de Gabès, École Nationale d'Ingénieurs, Gabès, Tunisia 3 Université Paris Est, ICMPE, SPC, PoPI team, UPEC, 2-8 rue Henri Dunant, 94320 Thiais, France. 4 Univ Paris Diderot, Sorbonne Paris Cité, ITODYS, UMR CNRS 7086, 15 rue JA De Baïf, France. 2 SUPPORTING INFORMATION SI1 MMT-MSA MMT-MA 1715 1634 2951 1013 2841 MMT-Na 3413 1641 1030 3624 Intensity (a.u.) 2881 1004 4500 4000 3500 3000 2500 2000 1500 1000 Wavenumbers (cm-1) IR spectra of MMT-Na, MMT-MA and MMT-MSA. 500 SUPPORTING INFORMATION SI2 MMT-Na MMT-MA MMT-MSA Intensity (a. u.) Quartz -98-008-0349 Montmorillonite - 98-016-1171 Kaolinite -98-003-0285 0 Illite - 98-016-6964 20 40 60 80 100 XRD patterns of MMT- Na, MMT-MA and MMT-MSA. SUPPORTING INFORMATION SI3 Lead was detected at the surface of the adsorbent MMT-MSA. The spectral 60-180 eV spectral regions from MMT-MSA after removal of Pb(II) displays Pb4f7/2-Pb4f5/2 doublet centred at 139.4-144.3 eV. These binding energies are consistent with Pb(II) from lead nitrate [1,2]. SUPPORTING INFORMATION SI4 The kinetic model of the first-order rate expression of Lagergren based on solid capacity is generally expressed by [3]: q= qe (1 − e−k1∗t ) (1) where, k1(1/min)is the pseudo first-order rate constant for the adsorption process, qe and q are the metal ion content that adsorbed per gram of MMT-MSA (mg Pb(II)/g) at equilibrium and at time t (min), respectively. The kinetic model of the pseudo-second-order Pb(II) adsorption is[4]: q= k2 q2e t 1+k2 qe t (2) where, k2(g/(mg.min)) is the rate constant of the adsorption process, qe and q are the metal ion content adsorbed per gram of MMT-MSA (mg Pb2 + /g) at equilibrium and at time t (min), respectively. Kinetic constants for Pb(II) adsorption onto MMT-Na and MMT-MSA. Samples MMT-Na MMT-MSA qexp 47.63 72.3 Pseudo-first-order qe K1 R² 55.49 0.0139 0.9506 73.45 0.0615 0.9271 Pseudo-second-order qexp qe K2 47.63 75.93 1.49708E-4 72.3 81.4 9.80406E-4 R² 0.916 0.887 SUPPORTING INFORMATION SI5 The Langmuir model and is given by [5]: q= 𝑞𝑚 ∗ 𝐶𝑒 ∗ 𝐾𝐿 (3) 1+(𝐶𝑒 ∗ 𝐾𝐿 ) where q (mg/g) is the adsorbed amount at equilibrium, Ce is the equilibrium concentration of the metal ions (mg/L), KL is Langmuir equilibrium constant (L/mg) and qm the maximum adsorption capacity (mg/g). The empirical Freundlich isotherm is obtained on the assumption that the sorption takes place on a heterogeneous sorbent surface. It is also applicable to multilayer sorption and is expressed by [6]: q= 𝐾𝐹 (𝐶𝑒 ) 1 𝑛 (4) where KF (mg 1−1/ n /g L 1/ n ) is the Freundlich constant and n is the heterogeneity factor. The KF value is related to the adsorption capacity; while 1/n value is related to the sorption intensity. [1] V. I. Nefedov, Y. V. Salyn and X. Keller, Zh. Neorg. Khimii1979, 24, 2564 [2] J. A. Taylor J.A., G. M. Lancaster and J. W. Rabalais, J. Electron Spectrosc. Relat. Phenom. 1978, 13, 435-444. [3] S. Lagergren, Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1-39 [4] Y. S. Ho and G McKay, Process Biochem.,1999,34, 451–465. [5] I. Langmuir, J Am ChemSoc, 1918, 40, 1361–1403 [6] H. M. F. Freundlich, Z Phys Chem., 1906, 57,385–470.