Final Research Paper - AOS-HCI-2011-Research
advertisement

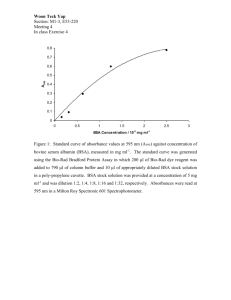
Determining the applicability of electrospun nanofibers as antibiotic delivery systems for the treatment of plant bacterial infections Varun Kulkarni & Erik Warnquist 1.0: Abstract Bacterial infections in crop plants are currently treated in highly inefficient and expensive manners. Current techniques involve completely coating the surface of infected plants with antibiotics that are potentially harmful to the environment. This can result in dangerous runoff that is not only hazardous to the environment but inefficient in effectively targeting bacteria. Our project utilizes the process of electrospinning to combine a common plant antibiotic, ampicillin sodium salt, with a widely available transport protein, bovine serum albumin (BSA), into one nanofiber. Particles of such nanofiber meshes have shown promise in penetrating cell walls; by targeting and penetrating specific afflicted cells, the treatment will greatly reduce the runoff into the environment as well as the overall cost of curing afflicted plants. We developed an 8.7 wt.% control solution in distilled water, with the components of the solute being 85 wt.% BSA and 15 wt.% polyethylene oxide (PEO). We also developed two solutions that incorporated ampicillin sodium salt: one solution dividing the 85 wt.% BSA into 42.5 wt.% ampicillin sodium salt and 42.5 wt.% BSA, and the other dividing the 8.7 wt.% into 33.3 wt.% ampicillin sodium salt, 33.3 wt.% BSA, and 33.3 wt.% PEO. Each solution was spun at a distance of 15cm from the grounded collector and with a voltage of 15kV. The solution was loaded into a bevel-tipped needle. Examination under a scanning electron microscope revealed that the fibers had significant globular formation, thus demonstrating that the solutions may not have been capable of legitimate matrix construction. Agrobacterium tumefaciens, a common plant bacterium, was grown on solidified agar and was subjected to samples of the different fibers. No bacterial death caused by the introduction of fibers was noted. We believe that the electrospinning process compromised the bioactivity of the ampicillin sodium salt and/or the ability of the BSA to act as a carrier protein, thus inhibiting effective treatment. For future trials we look to electrospin standardized fibers with a proper matrix construction, and then administer a prepared BSA-ampicillin sodium salt solution, perhaps through soaking the porous fibers. 2.0: Introduction The concept of electrospinning has recently gained much attention as a potential method of delivering bioactive agents, such as drugs that could be used to treat bacterial infections. Electrospinning is a process in which a solution is placed inside a capillary tube and is exposed to an electric field. The surface of the liquid becomes charged, and the power of the electric field is increased. The surface of the solution, in the shape of a hemisphere, is slowly pulled away from the capillary, creating a cone-like shape known as the Taylor cone. The power of the field is still increasing, and a point is reached where the electric charge overcomes the surface tension of the solution. A charged jet of fluid is released from 1 the Taylor cone, and lands on a grounded collector. As the fluid exits the cone, it lands on random places on the collector these random landing zones originate from the fact that the fluid within the cone is spinning around with different trajectories. This spinning, or “whipping,” process causes the solvent to evaporate, and the charged polymer fibers are left behind. The fibers are highly stretched and reduced in diameter, which results in a very high surface area to volume. The quality of the fibers is heavily dependent on the characteristics of the solution, such as viscosity and molecular weight (Frenot & Chronakis, 2003). Incorporating antibiotics into nanofibers is useful because of this high ratio, as it provides a large amount of drug coverage compared to the volume. However, adding a drug to a solution can considerably change its viscosity, surface tension, and conductivity, which may be problematic as varying any of these factors may restrict or impede the formation of fibers. According to Buschle-Diller et al., factors of the spinning solution that should be considered are, among other things, its miscibility (the ability of liquids to dissolve in any quantities or ratios), compatibility, and evaporation rate (2007). Miscibility is particularly important when a bioactive agent is being incorporated into a spinning solution, as a higher miscibility would result in a more homogenous solution and a greater chance of successfully forming fibers. Along with taking such factors into account, the location of the drug within the nanofiber also plays a crucial role in determining its release characteristics (Buschle-Diller et al., 2007). Other techniques have been developed in order to bypass the complexities of creating a solution with an antibiotic already incorporated within it. One such technique is the plasma treatment method. This technique involves the treatment of nanofibers with plasma to increase their surface adhesion. Plasma treatments with air or argon have been used in particular because of their ability to be dissolved or wetted by water. It is more convenient because it does not require producing a solution that retains the antibacterial properties of a drug while being able to form nanofibers. Once the nanofibers are formed, plasma treatment can be used to incorporate them with anti-bacterial properties. (Hyuk, Taek, & Park, 2 2009). The antibacterial properties of the fibers from this treatment are very effective: 99.999% of Staphylococcus aureus was killed after 4 hours of contact (Yao, Li, Neoh, Shi, & Kang, 2008). However, even with these techniques, a solute for the solution is still required. One proposed solute is albumin. Albumin is an abundant plasma protein that can be used as a solute to produce nanofibers. Using albumin has many advantages; its stability in a range of pH levels, it’s resistance to heat without damaging effects, and it’s biodegradability make it a strong candidate as a possible solvent. Also, it is neither toxic nor immunogenic, which is beneficial (Kratz, 2008). 3 Figure 1.0 Surface modification techniques for nanofibers (Hyuk, Taek, & Park, 2009) It is also important to examine the different aspects of bacteria, specifically A. tumefaciens, in order to determine the most effective method of combating them. Bacteria are prokaryotes, or microscopic single celled organisms that are found in every habitat on earth. They are only micrometers in length and come in a variety of shapes, including bacilli and cocci. The most adaptable and testable 4 bacterium for laboratory purposes is the Agrobacterium tumefaciens. The A.tumefaciens is a pathogenic bacterium that negatively affects the plant it inhabits. When infecting the plant it causes the disease known as the crown gall, a very economically significant disease because the effects it has upon plants calls for expensive treatments or removal that can further hamper the grower. The gall begins to develop because of the way the bacterium inserts its DNA into the ribosome of the plant, thus causing the overproduction of cytokinins, auxins, and opines. The auxins and cytokinins are responsible for the growth of the gall tumor and the opines serve as nutrients for the bacteria. After infecting the plant, cankerous tumors that deform the stem of the plant begin to appear. At the same time the metabolism of the plant starts to become impaired due to the combination of the DNA of from the A.tumefaciens and the infected plant (Collins, 2001). Specifically, the bacterium inserts a portion of its plasmid, which results in uncontrolled cell division and eventually in the formation of the crown gall typical of A. tumefaciens infections. Research also indicates that Agrobacterium can suppress the response of the plant immune system to develop a more efficient system of transformation and infection (Ditt, Nester, & Comai, 2005). A.tumefaciens is known to affect over 60 plant families throughout the world. The bacterium belongs to the Rhizobiacae family and is classified as Gram-positive. Furthermore the structure of the bacteria is the rod shaped and they grow aerobically within the plant without developing endospores. When the A.tumefaciens are applied to a culture containing carbohydrate substance with large amounts of extracellular polysaccharides the cultures will develop in fairly voluminous amounts with a slimy appearance according to laboratory observations (Collins, 2001). The bacteria A. tumefaciens can severely impair the plant it infects. However, this is not always the case. Some bacteria are in fact essential for the development of some plants because of the way they use nitrogen. Many bacteria are capable of extracting the nitrogen from the atmosphere and combining it with hydrogen from the soil to create ammonium, which plays a vital role in the balance of the soil and the ecosystem. Bacteria are an essential factor for the balance of nature, so it is essential to point out that 5 preventive antimicrobial treatments should only be used on bacteria that negatively impact their host plant (Knee & Nameth, 2007). Antibiotic use on plants contributes to approximately 0.1% of the total antibiotic use in the United States, reports Patricia McManus of the University of Wisconsin (2007). The majority of this amount is dedicated to fruit producing crops such as pears, apples, and peaches. Other food plants common to the usage of antibiotics are tomatoes, potatoes, peppers, and nectarines. Despite the recognition during the 1950’s and 1960’s that antibiotics could prove to be the miracle drug to cure all plant ailments, the movement has encountered issues. The sheer price of developing the quantities needed to treat whole acres has significantly outweighed the success antibiotics have been able to display throughout the years. Because of the pricing issues associated with the use of antibiotics, growers in many regions depend on whether based prediction systems that pin point the most efficient time to make use of the treatments. Secondly, due to the emergence of resistances to the drugs, problems have occurred in the treatment of plant bacterial infections. There are currently only two drugs registered by the EPA for safe usage on plants, Streptomycin and Oxytetracycline. In recent years groups have rallied around the idea that through the use of antibiotics on plants, the ability of life saving drugs to do their job in the future is significantly decreasing. Growers whose lives depend on the livelihood of their crops counter the argument with the idea that with the minimal amount of antibiotics used, no effect will take place (McManus, 2007). A. tumefaciens can be easily cultured through the use of LB broth. This broth can be used in either a solid induction method or a liquid induction method. One of the ideal strains of A. tumefaciens to culture is AGL-1. However, it should be noted that this strain cannot grow with the assistance of different antibiotics blended with the agar, which help repel other bacteria and overall contamination of the colony; there may be a slightly higher risk of sample contamination in growing AGL-1 (Flowers & Vaillancourt, 2005). The goal of this investigation was to develop a drug delivery system that incorporates an antibiotic (ampicillin sodium salt) and a carrier protein (bovine serum albumin) within a nanofiber. By 6 using nanofibers to administer drugs, the amount of antibiotics used would be significantly decreased. Furthermore, using nanofibers as a drug delivery system is advantageous as it can target specific infected locations rather than rather than rely on crude blanketing techniques. The bovine serum albumin, which is a hydrophilic molecule with a hydrophobic cleft, was ideal as a transport molecule not only because of its molecular structure but because of its hardiness; the molecule can withstand a pH range of 4-9 as well as temperatures up to 60 degrees Celsius without any significant change in structure. It was hypothesized that by blending the two components in a solution, the electrospinning process will have fused them together into one fiber without compromising bioactivity. Several solutions were prepared, with the solute components being BSA, ampicillin sodium salt, and polyethylene oxide (PEO), a common spinning agent, and the solvent being distilled water. The subsequent fibers were examined under a scanning electron microscope to observe fiber quality and fiber consistency. Also, samples of each fiber were taken and applied to an in-vitro culture of A. tumefaciens to determine their bioactivity. A series of fibers without an antibiotic component were used as a control to compare to the results of any experimental fibers. 7 3.0: Methodology 3.1: Solution Development A control solution was developed, composing of bovine serum albumin (BSA), polyethylene oxide (PEO), and distilled water (dH2O). The solution was composed with an 8.7 wt. % solute, which was further broken down into 85 wt. % BSA and 15 wt. % PEO. The solvent was dH2O. Two alternative solutions were also created, with each one incorporating ampicillin sodium salt, a common antibiotic for plant bacterial infections. The first solution divided the 8.7 wt. % solute into 42.5 wt. % BSA, 42.5 wt. % ampicillin, and 15 wt. % PEO. The second solution divided the solute into 33.3 wt. % BSA, 33.3 wt. % ampicillin, and 33.3 wt. % PEO. Bovine serum albumin was obtained from Sigma Aldrich, product number A7906. Ampicillin sodium salt was obtained from Sigma Aldrich, product number A0166. Both were refrigerated at a constant temperature of approximately 4 degrees Celsius. PEO was obtained from AOS. 3.2: Electrospinning Setup Each of these solutions was exposed to identical electrospinning parameters. Every solution was loaded into a 5mL syringe; the syringe was placed in a syringe pump. The needle used was a bevel-tipped needle, and the flow rate was set at a constant 0.2mL/hr. The distance between the tip of the needle and the grounded collector plate was 15cm. The solution was spun at 15 kilovolts. The collector plate was made out of copper and was covered with aluminum foil; the foil provided a plane on which the fibers could be collected. Half of the electrospinning trials were spun with Flouroglide® sprayed onto the aluminum foil. Flouroglide® is a Teflon like substance that eases the removal process when collecting the fiber for bacterial trials. The Flouroglide® was sprayed onto the foil then dried via the use of a heat lamp for 8 approximately 5 minutes. This allowed the spray to dry and thus not mix with the BSA solutions when electrospinning. 3.3: Scanning Electron Microscopy The resultant fibers were examined under a scanning electron microscope, which was located in Janelia Farms research center (due to scheduling conflicts, the fiber from the solute composed of 42.5 wt. % BSA, 42.5 wt. % ampicillin, and 15 wt. % PEO could not be examined). The fibers were examined then two pictures were taken of each fiber respectively. Each picture was conducted at different magnifications and was taken as to be representative of the fiber structure on the whole. Magnification levels, pixel size, and relevant measurements of structures were recorded into appropriate laboratory notebooks. 3.4: Bacterial Preparation Solid agar plates were prepared using a dry agar solution from Scholar Chemistry. The agar was combined with water in the ratio of 23.3 grams of agar to one liter of dH2O. Large stock supplies of agar were prepared, autoclaved to remove unwanted bacterial growth, then stored at 8 Celsius in the refrigerator for later use. The cooled agar solution was then heated on a hot plate. A sterile work environment was prepared through the use of 10% bleach on lab surfaces. Then a Bunsen burner was lit to circulate air and prevent the landing of new bacteria onto the agar. The solution was then poured into the Petri dish, while keeping the top of the Petri dish at a 45 angle for further bacterial growth protection. The agar was allowed to re-solidify, usually via a 10-25 minute waiting period. Once the agar was solid, a solution consisting of Luria Broth (LB) and dH2O was prepared in the ratio of 35 grams of solid LB agar base to one liter of dH2O. A 100 ml solution was prepared using 3.5 grams of the LB agar base. Agrobacterium tumefaciens from Carolina® was then removed from refrigeration. An inoculating loop removed from sterile packaging was the swabbed across the surface of the A. tumefaciens nutrient agar 9 and swirled around in the LB solution. The other non-used end of the inoculating loop was then used to remove more bacteria and was also swirled around in the LB solution. A sterile L-shaped spreader was then immersed in the LB solution now impregnated with the A. tumefaciens. The spreader was then removed from the solution and spread across the surface of the agar, making sure to cover the entire surface. The Petri dish was subsequently closed then sealed with Parafilm® for the control sample. For the trials using the electrospun nanofibers the bacteria was applied to the Petri dishes in the same fashion. Prior to sealing however, these plates were treated with four respective treatments. Treatments were prepared by first pealing the fibers from the aluminum foil through the careful use of tweezers and scalpels. Once removed the fibers were cut into relatively similarly sized two dimensional pieces. Each piece was approximately a 0.5 cm by 0.5 cm square. On each plating four treatments were placed in a square formation (see below). Figure 3.4: Example of plating method with fibers located on the surface For each trial two control fiber treatments of the same batch were used on the plate for two of the spots. For the other two spots two treatments of the two trial concentrations were used (of the same batch). Four trials of two dishes each were used all together. 3.5: Data Recognition To examine the results for bacterial death, the process of region of inhibition was chosen. In this method the spread of bacterial death around the treatment it measured. The treatments were placed far enough apart in the hopes that the regions would not intersect thus further disturbing the data. The regions 10 were created, their respective diameters were measured. These diameters were compared to those of the control and between trial groups. 4.0: Results 4.1: Fiber Images Spun on: February 15th, 2010 Solution: 8.7 wt% control fiber: 85 wt. % BSA, 15 wt. % PEO Magnification: 2.61 x 103 Sample #: 1 Figure 4.1.1 Figure 4.1.1 is the first in a series of scanning electron images of the fibers that were developed. The solution that the fiber was created from, an 8.7 wt. % solute with 85 wt. % BSA and 15 wt. % PEO, was a control as it does not incorporate ampicillin sodium salt. The image shows significant globular formation, with clusters of spherical globs heavily concentrated around several narrow, thin strands of fiber. The thin strands are characteristic of the fibers generated from a pure PEO solution, so it was determined that the globular formations were composed of electrospun BSA. The overall lack of the development of a consistent BSA fiber is not favorable, in that inconsistent globs are indicators of a structurally unstable mesh. However, in this context of drug delivery, the globular formations may be beneficial, as they may have the potential to carry the ampicillin molecules within the fiber. 11 Spun on: February 15th, 2010 Solution: 8.7 wt% control fiber: 85% BSA, 15% PEO Magnification: 1.15 x 103 Sample #: 1 Figure 4.1.2 Figure 4.1.2 shows a different sample of a fiber of the same composition as Figure 4.1.1. The conclusions drawn about the fiber from Figure 4.1.1 are supported in this image. The globs of BSA still seem to follow the thin strand of PEO fibers, although not with enough consistency to form a clear nanofibrous mesh. This sample also lacked any continuous fiber composed of BSA. Spun on: February 15th, 2010 Solution: 8.7 wt% control fiber: 85% BSA, 15% PEO Magnification: 1.19 x 103 Sample #: 2 Figure 4.1.3 Figure 4.1.3 is another image of a sample of the same control solution. This image shows a clearly defined clustering of the globular protein formations around specific patches of the PEO mesh. However, there is still no continuous and consistent fiber. 12 Spun on: February 15th, 2010 Solution: 8.7 wt% control fiber: 85% BSA, 15% PEO Magnification: 8.18 x 103 Sample #: 2 Figure 4.1.4 Figure 4.1.4 is a magnification of a patch of the previous sample. It is clear to see that the locations of each glob of BSA are somewhat influenced by the position of the PEO meshes, but that influence is not enough to completely create a fiber with a PEO core and BSA globs as an external layer. Spun on: February 15th, 2010 Solution: 8.7 wt% control fiber: 85% BSA, 15% PEO Magnification: 3.63 x 103 Sample #: 2 Figure 4.1.5 The figure on the previous page is another image of the same sample. Again, the trends outlined in the previous images are consistently being supported in this image. The globular formations seem to share a collinear pattern similar to that of the PEO mesh that is closest to them, but the correlation is not strong enough to develop a neat fiber. 13 Spun on: March 9th, 2010 Solution: 8.7 wt% control fiber: 85% BSA, 15% PEO Magnification: 1.21 x 103 Sample #: 3 Figure 4.1.6 As seen with the previous control fibers, very little fibrous formation was evident in this updated sample. Nearly no connection matrices are visible between the globs of BSA. They seem to be conglomerating around the “fibers” of PEO. However, even with the assistance of the spinning agent, very little fibrous construction can be observed in this sample. Spun on: March 9th, 2010 Solution: 8.7 wt% control fiber: 85% BSA, 15% PEO Magnification: 8.83 x 103 Sample #: 3 Figure 4.1.7 The large globular structures appear to be BSA, as PEO is a highly stable and distinct fiber when spun. The average size of the globs appears to be approximately 897.1 nm. Accompanying the globs are shredded forms of the PEO. Essentially, this shows no distinct fiber formation; the image seen is a clustering of the solution prepared into the electrospinning machine. 14 Spun on: March 9th, 2010 Solution: 8.7 wt% test fiber: 33.3% BSA, 33.3% PEO, and 33.3% ampicilin Magnification: 1.56 x 103 Sample #: 4 Figure 4.1.8 In this image, it seems that the globs seem to be congregating more heavily around the portions of PEO. This could be due to the increase of the concentration of PEO within the solute. This is logical, as an equal wt. % of BSA and PEO within the solution would most likely yield a more consistent fiber, even if the fiber is not regular. Another important observation is the smeared effect of the globs of the BSA. It is possible that the addition of the ampicillin into the mix somehow morphed what was occurring in the BSA and stretched the globs, although this may also be a side effect of balancing the wt. % of the BSA and the PEO. The fibers in this image also seem to have protruding shards of what may be ampicillin (the shape is not similar to either spun BSA or PEO). The difference in the texture of the fibers, as compared to the previous images, may denote a coating of the fibers with the morphed BSA. It is important to note that the globs seem to be located in significant clusters, which are highlighted by the circles; the clustering is similar to the correlation between PEO concentration and BSA conglomeration seen in the previous images. Overall, the addition of ampicillin in the solution as well as the balance between the 15 BSA concentration and the PEO concentration have yielded favorable results; this image shows more distinct fibers that are potentially lined with ampicillin and coated with BSA. Optimally, such a fiber may receive protection from the BSA coating and remain bioactive from the ampicillin shards. Spun on: March 9th, 2010 Solution: 8.7 wt% test fiber: 33.3% BSA, 33.3% PEO, and 33.3% ampicilin Magnification: 5.50 x 103 Sample #: 4 Figure 4.1.9 At first glance this image has a striking resemblance to portion B of figure 1.0 in which the surface graft polymerization process is described. This image is promising because at a more magnified view, 5.50 x 103 magnification, it can be seen that there is indeed some fiber formation. Although the bioactivity of such structures cannot be confirmed, it does appear that there is a PEO base fiber with globs of BSA/ampicillin connected in branching formations. This is further supported by the measurement of the glob, 935.7 nanometers in length, which is highly similar to the sizing of the BSA glob measured in sample 3 (Figure 4.1.7.). 16 4.2: Bacterial Results and Analysis Figure 4.2.1 The pictures above(figure 4.2.1) is an images of a colony of Agrobacterium tumefaciens being treated with a shard of a fiber that was created from a solute of 15 wt. % PEO, 42.5 wt. % BSA, and 42.5 wt. % ampicillin (not shown in the image above). The rationale for such a solution is that the resultant fiber will contain equal concentrations of BSA and ampicillin, in such a way that the amount of antibiotic in the solution is maximized while preserving the 15 wt. % PEO as a spinning agent. The treatment revealed some mixed results. The colony is obviously affected by the treatment, as the texture and the color of the bacteria definitely changed (the colony on the right was left untreated as a control; the color of the treated and the untreated bacteria clearly differs). The shard that was used as a treatment also seemed to be directly consumed by the bacteria, as it is completely coated with bacteria. No bacterial death was observed, and no zones formed. The growth of the bacteria over the treatment actually indicates that the nanofiber was ineffective in killing/preventing bacterial growth. In other words, this evidence goes against the hypothesized bioactivity of the fiber. The color change may or may not be a sign of bioactivity, as color change can result from a variety of causes, such as a fungal contamination. As 17 there was no way to confirm whether or not the color change is a result of the bioactivity of the fiber, the test was inconclusive. The trend observed here is representative of all the other data. In fact, to put the images of the plates covered with thriving bacteria would be useless. The results found on the agar trials were so decisive that they did not even warrant a picture. Neither concentration of fibers provided any bacterial death. This would demonstrate an error in process of methodology that will be discussed later in the discussion section (5.0). Furthermore even the positive control we conducted provided no results. We created a base control solution of 8.7 wt% 85% BSA and 15% PEO then immersed sterile filter paper circles of 0.5 mm radius into the solution. We prepared an agar via the L-shaped spreader then placed the circles onto the bacteria in the same formation we did with the fiber treatments. After placement into the incubator and 2 day waiting period, once again there was absolutely no bacterial death. This demonstrates problems with the overall solution preparation if not others. 5.0: Discussion The scanning electron microscope images of the different fibers were obtained from the Janelia Farms Research Center. The fibers that were examined were all spun at a constant 15 kilovolt charge and at a distance of 15 centimeters between the tip of the syringe and the grounded collector. The concentrations and contents of the solutions were modified for different experimental fibers. Control fibers were created from the control solutions with a solute of 8.7 wt %, which was further broken down into 85 wt. % bovine serum albumin and 15 wt. % polyethylene oxide. The solvent was distilled water. Trial fibers were composed from two trial solutions, with the solute remaining a constant 8.7 wt %: one was 42.5 wt. % BSA, 42.5 wt. % ampicilin sodium salt, and 15 wt. % PEO, and the other was 33.3 wt. % BSA, 33.3 wt. % ampicillin sodium salt, and 33.3 wt. % PEO. These two trial solutions were spun at the 18 same voltage and distance from the grounded collector as the control. These fibers were collected on aluminum foil and then examined via the SEM. The images of the fibers can be seen in Figures 4.1.1 through 4.1.9. Analysis for each individual fiber is provided beneath the images. However, when looking at the general fiber construction, overarching conclusions can be drawn. Consistent throughout the solutions, there is very little fibrous construction. Instead, globs of BSA with with relatively minute PEO connection fibers are apparent. This may have been due to a lack of sufficient PEO in the solution as a polymerizing/fiber constructing agent, the high viscosity of the solution, or other experimental/ambience flaws, such as humidity, stirring techniques, etc. In any case, the fibers did not exhibit the characteristics of an optimum and stable fiber, such as consistency and lack of beading. Despite this problem of legitimate fiber construction, the fibers did have the potential to carry antibiotics. Figure 4.1.8 and figure 4.1.9 provide the best insight into the formation of the fibers. Figure 4.1.8 shows how the addition of more PEO and ampicillin sodium salt into the solution affected the fiber by stretching out the globs of BSA into more of a smear. It is believed that this may have occurred primarily due to the addition of ampicillin, as PEO is known to form only narrow, long fibers. Such an effect is unlikely to affect the BSA globs. Furthermore, PEO is not likely to interfere with the spinning of any other component of the solution, as it is such a stable spinning agent. The stretched out globs may be a result of modifying the solution with the addition of ampicillin sodium salt. Figure 4.1.8 also shows significant clustering of globs, as indicated by the circles. This again may have occurred because of the ampicillin or because of the balance in concentration between the BSA and PEO. By having them in equal amounts, the PEO would have been more likely to create connections between the globs and perhaps bring them into contact. Figure 4.1.9 gives a magnified image of the same sample. This figure shows a construction very similar to that of portion B of Figure 1.0 (the surface graft treatment). The similarity indicates that the image may indeed show a successfully treated fiber (albeit through a blend solution method rather than through surface graft treatment). The differences in texture, along with a comparison with the other control images, led to the conclusion that the fiber in the image 19 may be a PEO base fiber that incorporates shards of ampicillin sodium salt and is coated by the BSA. Such a combination is favorable because of the stability of the base and the hardiness of the coating. It should be noted, however, that such images do not indicate the status of the fibers’ bioactivity. This is a promising find and shows that for later studies, it may be of use to keep the polymer base and the other components in equivalent ratios. In order to test the efficacy of the treatments Petri dishes were plated with A. tumefaciens. The dishes were plated via the lawn method it which the bacteria was introduced into a Luria broth solution. This solution was then spread across the surface of the agar. This was done so that the bacteria would grow consistently across the plate. Directly after being plated, 1 cm by 1 cm square portions of the fibers were applied onto the plate as shown in figure 3.4. After this was done the plates were placed in the incubator at 25 degrees Celsius. After remaining in the incubator for 48 hours the plates were removed. Similar to image 4.2.1 treatments had absolutely no ability to kill bacteria. Despite some changes to the color of the bacteria, the bacteria lived on successfully. This trend continued with all the trials of both concentrations. Of the 10 agar dishes that were prepared, no trials demonstrated any sort of bacterial death. We proposed several hypotheses to describe why the fiber was unsuccessful in killing and preventing the growth of A. tumefaciens. It was first hypothesized that the ampicillin sodium salt had lost its bioactivity in the electrospinning process; exposure to 15kV may have altered the structure of the antibiotic, thus rendering it ineffective. Another hypothesis stated that the colony of A. tumefaciens, which has a rapid growth rate, may have a grown over the antibiotic after it had taken effect. This was a possibility, as the effects of the antibiotic were not observed until 2 days after its application (a regular drug-laden fiber will release approximately 99% of its drug in 4 hours). A third hypothesis stated that the problem was with the original solution; the ampicillin may have lost its bioactivity before the electrospinning process, or may have reacted unfavorably with the BSA or PEO. We also considered the notion that the strain of bacteria that was being used may be an ampicillin-resistant strain. Several 20 different tests were conducted to isolate the error to one of these variables. To eliminate the possibility of the A. tumefaciens re-growing over our treatment, snapshots were taken 4 hours after the application as well as a day after the application. Neither observation showed any initial zone of inhibition or re-growth. As a result, we were able to eliminate the notion that the growth rate exceeded the effects of the antibiotic. To determine whether the loss in bioactivity occurred during the electrospinning process, a trial of positive controls was established. The idea of this trial was to see if the ampicillin sodium salt could successfully kill and/or form a zone of inhibition in the culture before it was exposed to the high voltages associated with electrospinning. To run the positive control, several cultures of A. tumefaciens were first prepared, along with the control solution and the solution that had a balanced solute. We soaked separate sets of filter paper in both solutions, and placed them in different locations in the different cultures. Neither the control nor the experimental solution showed any bioactivity or effectiveness in handling the bacteria. This demonstrates that the bioactivity may have been compromised even before the electrospinning process, which indicates that either the bacterium is ampicillin-resistant or the solution is interacting unfavorably. The source of error between these two options has not yet been confirmed. It is also important to note that this testing did not rule out the possibility of denaturing through the electrospinning process. In conclusion, nanofibers still hold promise in integrating ampicillin sodium salt and bovine serum albumin into a feasible drug delivery system. Based on the experimental scanning electron microscope images, a basic fiber matrix can still be developed, with BSA and ampicillin as additions to the base structure formed by the PEO. However, the basic matrix outline was only seen when the components of the solute had a balanced concentration, which indicates that the solute must not be dominated by one substance if it entails multiple agents. Also, the structural stability and the effectiveness of the ampicillin sodium salt after being subject to the electrospinning process has not been examined; it is possible that the extreme voltages may have affected the ability of the antibiotic to kill/prevent the growth of A. tumefaciens. 21 For the future we plan to pursue to the ability of BSA to assist nano-scale drug delivery systems. The best method for improvement would be to modify how the fibers are prepared in order to facilitate the bacterial killing process. This can take on two pathways: either to focus on the biocompatibility of the solution prior to spinning or to develop a structurally stable fiber first and then treat it to add the antibiotic. Such a method is advantageous in that the sensitive antibiotic is able to bypass the high voltage of the electrospinning process. If the solution can be seen to exhibit antibacterial properties prior to spinning, then techniques such as surface graft polymerization will certainly hold promise in creating a stable and bioactive fiber. Research also indicates that fibers, due to the nature of which they are created, are responsive to electromagnetic fields. It may be possible to increase the targeting abilities of the nanofibers by manipulating them with such fields, thus increasing their overall efficiency. 22 References Buschle-Diller, G., Cooper, J., Xie, Z., Wu, Y., Waldrup, J., & Ren, X. (2007). Release of antibiotics from electrospun bicomponent fibers. Cellulose, 14(6), 553-562. Collins, A. (2001) Agrobacterium tumefaciens. Department of Plant Pathology, University of North Carolina State. Retrieved September 19, 2010 from: http://www.cals.ncsu.edu/course/pp728/Agrobacterium/Alyssa_Collins_profile.htm Ditt, R. F., Nester, E., & Comai, L. (2005). The plant cell defense and Agrobacterium tumefaciens. FEMS Microbiology Letters, 247, 207-213. Flowers & Vaillancourt (2005). Agrobacterium tumefaciens-mediated transformation of Colletotrichum graminicola and Colletotrichum sublineolum. Journal not given. Frenot, A., & Chronakis, I.S. (2003). Polymer nanofibers assembled by electrospinning. Current Opinion in Colloid and Interface Science, 8(1), 64-75. Hyuk, Y.S., Taek, G.K., & Park, T.G. (2009). Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Advanced Drug Delivery Reviews, 61(12), 1033-1042. Knee, M. & Nameth, S. (2007) Horticulture and Crop Science: Bacteria. The Ohio State University, Horticulture Department. Retrieved Septermber 12, 2010 from: http://www.hcs.ohiostate.edu/hcs300/bact.htm Kratz, F. (2008). Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. Journal of Controlled Release, 132(3), 171-183. McManus, P. (2007) Antibiotic Use in Plant Disease Control. Fruit Pathology: University of WisonsinMadison. Retrieved September 13, 2010 from: http://www.plantpath.wisc.edu/fpath/antibioticuse.htm 23 Yao, C., Li, X., Neoh, K.G., Shi, Z., & Kang, E.T. (2008). Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. Journal of Membrance Science, 320(1-2), 259-267. 24