SignificantFigures2
Significant Figures
Significant Figures
Every time a measurement of some physical quantity is made, such as the mass of an object or the volume of a solution, there is some degree of uncertainty in the measurement .
This uncertainty i s due to various factors, such as, the accuracy of the device and the skill of the person doing the measuring.
The recorded value for a measurement should give an indication to the reader of its reliability .
Significant Figures
Scientists have adopted a method of reporting the reliability of measurements by using what is known as
significant figures
.
In this procedure all numbers that are known with certainty are recorded, plus a last number that has been estimated (some uncertainty).
All the numbers in such recorded measurements are called SIGNIFICANT FIGURES .
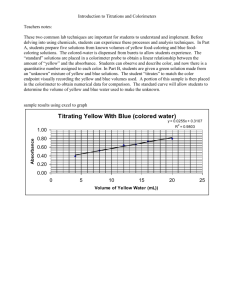
The figure shows how the same volume of liquid, when measured in Burets of different degrees of possible accuracy will result in different numbers of significant figures.
A B C
The first buret is a 50 mL graduated buret that has calibration marks every
10 mL.
A
Buret B is a 50 mL graduated buret similar to A, but it also contains marks every 1 mL.
A
The Buret C has the best possibility of giving the most accurate readings since it is a 50 mL graduated buret that has calibration marks every 0.1 mL .
A B C
Let us first look at the volume of the liquid in Buret A.
The liquid level is between the 30 mL and 40 mL calibrated marks. Since the level appears to be halfway between these two values, we then record the measurement as
35 mL.
A
However, the volume could be as small as 34 mL or as large as 36 mL.
The uncertainty in the first measurement is one milliliter above
(+) or below (-) our estimated value or we can say that the measurement is uncertain by +/- 1 mL.
The recorded value of 35 mL, thus has two significant figures (the number of certain numbers plus one estimated!); the "3" is certain, but the
"5" is an estimate.
A
In Buret B, the same volume of liquid is found to be between 35 mL and 36 mL.
However, if we look very closely we can see that the level appears to be slightly less than halfway between these two values.
By using this graduated buret we can be certain that the volume is between 35 mL and 36 mL and we can also estimate its value to the nearest one-tenth of a milliliter (+/- 0.1 mL).
We would report that the measured volume is 35.4 mL.
However, we must realize that this measurement is uncertain by +/- 0.1 mL, and the volume could be as low as 35.3 mL or as high as 35.5 mL.
The reported value of 35.4 mL has three significant figures since the "3" and "5" are certain and the "4" is an estimate.
When we observe the same volume in the last 50 mL graduated buret, we are now able to estimate to the nearest +/-
0.01 mL.
We see that the liquid's level is between the 35.4 mL and 35.5 mL marks. Also, we can see that the level is approximately halfway between the 35.4 mL and 35.5 mL marks.
Thus, the volume is recorded to be 35.45 mL. The measurement 35.45 mL is said to have four significant figures.
In summary, if we look at any reported measurement, the uncertainty is assumed to be
+/- one unit in the last recorded number .
For example, if a report has a mass of an object as 11.6 grams, then it has three significant figures and the measurement has an uncertainty of
+/- 0.1 of a gram.
If 11.62 grams is reported for an object, then the measurement has four significant figures and the uncertainty of the measurement is
+/- 0.01 of a gram.
Some other examples are as follows:
Measurement The numbers that are certain
The numbers that are uncertain
Number of significant figures
(1) 36 mL
(2) 36.8 mL
"3"
"3" and "6"
(3) 4536 meters "4", "5", and "3"
(4) 0.052 gram "5"
(5) 95.00 cm
(6) 4.3
x 10
6 cm
"9", "5", and "0"
"4"
"6"
"8"
"6"
"2"
"0"
"3"
2
3
4
2
4
2