Experiment 1F-2 MEASUREMENTS IN THE CHEMISTRY LABORATORY II
advertisement

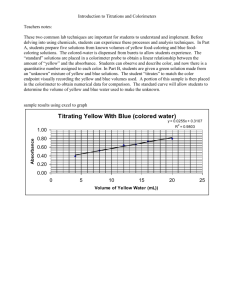
Experiment 1F-2 FV 12-5-14 MEASUREMENTS IN THE CHEMISTRY LABORATORY II PURPOSE: The purpose of this experiment is to become familiar with the common types of laboratory glassware and equipment, make observations, and analyze data. This is a continuation of last week’s lab, Expt. 1F-1. PRE-LAB: Before lab, complete the pre-lab exercises on page 1F2-5. Bring your pre-lab, your safety goggles, and this handout with you to lab. Since you will also be doing Excel calculations in lab, bring your laptop. PROCEDURE: Equipment demonstrations: Your instructor will demonstrate how to use a buret. For accurate results, it is important to use a buret properly. Part A: Determination of the Density of a Copper Solution Using a Buret 1. Using a top-loading balance, determine the mass of a small plastic bottle with its cap (don’t forget to tare the balance first). It is not necessary that the bottle is completely dry on the inside as long as it is capped. The bottle must be dry on the outside. 2. With a clean 100 mL beaker, obtain about 80 mL of the prepared aqueous copper solution from the labeled container in the room. 3. Fill a 50 mL buret with the copper solution making sure there are no air bubbles in the buret tip. The liquid level in the buret should start between 0 and 5 mL. 4. Record the initial buret reading with units and the correct number of significant figures. Buret volumes should be read to the hundredths place in volume (for example, 1.26 mL). 5. From the buret, add about 5 mL of the copper solution to the plastic bottle. The amount does not need to be exactly 5.00 mL as long as you know what you added. Cap the bottle tightly. 6. Record the final buret reading. 7. Weigh the capped bottle on a top-loading balance and record the mass. 8. Repeat steps 5-7 for three more different volumes (for example, add 8, 12, and 15 mL). The bottle does not need to be emptied between each addition. It’s only important that you know the volume of solution added from the buret and its corresponding mass. The buret does not need to be refilled (unless necessary). 9. When you are done, drain the copper solution from the buret into the bottle and save the copper solution for Part B. Part B: Reclaiming of the Copper 1. Pour about 50 mL of your copper solution from Part A into a 150 mL beaker. 2. Cut a small piece of steel wool (about the size of a silver dollar). Spread out the steel wool to increase its surface area. 3. Add the steel wool to the beaker and stir gently with a stirring rod. 4. To speed up the reaction, add 1-2 drops of 2 M hydrochloric acid (HCl). 5. Record your observations after a few minutes. 6. Discard the solid/liquid wastes in the bucket in the instructor’s hood. Part C: 1. 2. 3. Penny Masses Obtain 4 dry pennies from the front of the room. Tare an empty weigh boat on an analytical balance. Weigh each penny individually, after taring each time. Also record the year of each penny. Return the pennies. Clean-up: 1. All metal wastes from this lab (solid or liquid) should be disposed in the bucket in the instructor’s hood. 2. Rinse all glassware. Burets are typically stored (after cleaning) in the buret clamp upside down with the stopcock open to allow it to drain/dry. 3. Clean up and organize your lab station. Start working on your calculations. 1F2-1 Name ________________________________________ Section ______________________ Partner _______________________________________ Experiment 1F-2 MEASUREMENTS IN THE CHEMISTRY LABORATORY II DATA SHEET: Report all values with units and the proper number of significant figures. Part A: Determination of the Density of a Copper Solution Using a Buret * Initial Mass of Plastic Bottle (with cap) = ________________________ * Initial Buret reading = ____________________ Trial 1 Final Buret reading = _____________________ ~ 5 mL amount Final Mass of Plastic Bottle (with cap) + Cu solution added = ________________________ Total Volume of Cu solution added (Trial 1) = ________________________ Total Mass of Cu solution added (Trial 1) = ________________________ Trial 2 Final Buret reading = _____________________ ~ 8 mL amount Final Mass of Plastic Bottle (with cap) + Cu solution added = ________________________ Total Volume of Cu solution added (Trial 2) = ________________________ Total Mass of Cu solution added (Trial 2) = ________________________ Trial 3 ~ 12 mL amount (Note: this is the TOTAL amount added so far subtract initial values above* from final values) Final Buret reading = _____________________ Final Mass of Plastic Bottle (with cap) + Cu solution added = ________________________ Total Volume of Cu solution added (Trial 3) = ________________________ Total Mass of Cu solution added (Trial 3) = ________________________ Trial 4 ~ 15 mL amount Final Buret reading = _____________________ Final Mass of Plastic Bottle (with cap) + Cu solution added = ________________________ Total Volume of Cu solution added (Trial 4) = ________________________ Total Mass of Cu solution added (Trial 4) = ________________________ Part B: Reclaiming of the Copper Observations after mixing the copper solution and steel wool for a few minutes: Did a chemical reaction occur? What was produced? 1F2-2 (what changed?) Part C: Penny Masses Penny Mass Penny Year 1 2 3 4 Average Penny Mass = _______________________________ Standard Deviation of Penny Mass = ___________________________ (can be calculated on Excel or calculator) DATA ANALYSIS: 1. Summarize your Part A data below for each trial, then construct a hand-graph of mass vs. volume (y vs. x). Label the axes (with the quantity name and units). Draw a best-fit straight line through the data. x y Total Volume of Cu solution added (mL) Total Mass of Cu solution added (g) Trial 1 2 3 4 50 45 40 35 30 25 20 15 10 5 0 0 5 10 15 20 1F2-3 25 30 35 40 45 50 2. Based on your graph, determine the slope of your best-fit line (i.e., rise over run, or y over x). Show your work below. Based on your hand-graph, what is the density of your copper solution? _______________________ g/mL The actual value for the concentration of the aqueous copper solution is 1.02 g/mL. Determine the % error compared to this value. 3. Using the data in #1 above, construct an Excel spreadsheet and graph of mass vs. volume (y vs. x) – same as your hand-graph. Create a trendline for the data showing the equation and R2 value on the graph. Give the equation of the trendline: ____________________________________________________ Give the R2 value: ______________________ An R2 value (known as the correlation coefficient) close to 1.000 indicates a linear relationship between the x and y data. Based on your Excel graph, what is the density of your copper solution? ______________________ g/mL Was this value similar to what you obtained from the hand-graph? ______________ Make sure your columns and axes are labeled properly (with name and units). Your spreadsheet and graph should fit on 1 printed page, and your name should be on the spreadsheet. Submit your spreadsheet and graph with your lab report. 4. In last week’s lab (Ex. 1F1), you saw the following chemical reaction occur. Balance the reaction. One coefficient is provided. _____ Cu (s) + _____ HNO3 (aq) _____ NO2 (g) + _____ Cu(NO3)2 (aq) + 2 H2O (l) For every 1 mole of copper metal, how many moles of HNO3 are needed to react with it? ____________ Give the names for the following compounds and determine whether they are molecular or ionic compounds. Compound Name HNO3 (aq) NO2 (g) Cu(NO3)2 (aq) 5. Based on your Part C data, do all pennies have the same mass? Was there any trend between penny date and mass? Explain with your data. 1F2-4 Molecular or Ionic? Name ________________________________________ Section ______________________ Experiment 1F-2 MEASUREMENTS IN THE CHEMISTRY LABORATORY II PRE-LAB EXERCISES Being able to analyze and report data is important in many areas, including the military. One of the most common tools for this is a spreadsheeting program, such as Microsoft Excel. If you have never used Excel, go the Excel Tutorial on the Plebe Chemistry website to learn about Excel. You may also obtain assistance from your instructor or in the Chemistry Resource Room (Michelson 100). http://www.usna.edu/ChemDept/plebeChem/tutorials.php You must complete this assignment individually, from a blank Excel worksheet. For this pre-lab, use Excel to complete the following: 1. Create a spreadsheet with the following data: 2. Construct a graph of y vs. x. Label the axes. 3. Include the trendline and R2 value with the graph. 4. Print the spreadsheet and graph on one page to submit. 5. Include your name on your spreadsheet. An example spreadsheet and graph are included below. Note the formatting. Midn 4/C Smith 8-Aug-10 Glucose Concentration, mM 0.0 2.0 4.0 6.0 8.0 10.0 Experiment 1A Measured Absorbance, A 0.002 0.150 0.300 0.477 0.570 0.704 1F2-5 Data: x 1.22 2.55 3.00 4.04 y 5.51 7.66 9.00 12.31