C .elegans
advertisement

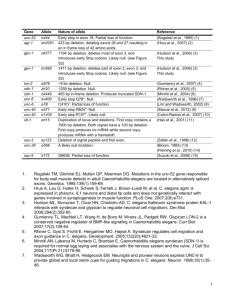
Victoria Wei Need Taken from Rajput AH, Offord KP, Beard CM, Kurland LT. Epidemiology of parkinsonism: incidence, classification, and mortality. Ann Neurol. 1984;16:278-282. Figure 1 The amount of Parkinson’s disease cases per 100,000 people in the United States as age increases Knowledge Base Parkinson’s disease is a brain disorder involving the nerves. Figure 2 The effects of Parkinson’s disease http://www.spinstudios.co.uk/sa/pa3.jpg Knowledge Base http://www.wormatlas.org/handbook/fig.s/IntroFIG6.jpg Figure 3 The life cycle of C. elegans Knowledge Base Lipofuscin is an auto- fluorescent age pigment which is found in people with neurodegenerative diseases. (Gray, et. al., 2005) http://www.innovitaresearch.org/news/res/06042501_01.jpg Figure 4 Lipofuscin in neurons of the human brain. Knowledge Base Centrophenoxine is an anti-aging medicine which slows the accumulation of lipofuscin. Schneider, et. al. (1977) http://commons.wikimedia.org/wiki/File:Centrophenoxine.svg Figure 5 Molecular structure of centrophenoxine Literature Review Caldwin, et. al. (2008) Figure 6 Figure 7 Both images taken from Caldwin, Guy A.; K.A. Caldwell. “Traversing a wormhole to Combat Parkinson’s disease.” Disease Models and Mechanisms. Volume 1. pp.000000. 2008. Literature Review Sutphin, et. al. (2009) Figure 8 Auto fluorescent pigments present in Day 4 and Day 8 C. elegans Sutphin, George; M. Kaeberlein. “Measuring Caenorhabditis elegans Life Span on Solid Media” JOVE. 2009. Literature Review Gerstbrein, et. al. (2008) Figure 9 Fluorescence of the C. elegans using the lipofuscin as a biomarker for health span. Gerstbrein, Beate; G. Stamatas; N. Kollias; M. Driscoll. “In viv spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Literature Review Application of centrophenoxine to the C. elegans decreased the rate of lipofuscin accumulation by an average of 41.3%. (Shulkin, et. al., 1978) Figure 10 Fluorescence of the C. elegans using the lipofuscin as a biomarker for health span. Shulkin, D.J.; B.M. Zuckerman. “Spectrofluorometric analysis of the effect of centrophenoxine on lipofuscin accumulation in the nematode C. elegans.” Age. Volume 5. Pp. 50-53. 1982. Purpose The purpose of the experiment is to observe the effects of centrophenoxine on the restriction of Parkinson’s disease symptoms in C .elegans Hypothesis Null- the symptoms of Parkinson’s disease will remain the same with or without the application of centrophenoxine. Alternate- the symptoms of Parkinson’s disease will lessen with the application of centrophenoxine. The Effects of Centrophenoxine on the development of Parkinson’s disease in C. elegans C. elegans obtained from the Caenorhabditis Genetics Center- N=80 Wild type C. elegans: N=40 Given centrophenoxine N=20 Control N=20 ham-1(ot339) C. elegans: N=40 Given centrophenoxine N=20 6.8 mM centrophenoxine will be applied to the Nematode Growth Media for 21 days. Use of 4',6-diamidino-2-phenylindole (DAPI) to observe the amount of auto fluorescent pigmentlipofuscin- in C. elegans. GFP filter will also be used. Statistical analysis with SPSS Control N=20 Protocol C. elegans are grown in petri dishes containing Nematode Growth Media (NGM) from Carolina Biological and fed U.V. killed Escherichia coli. Picture by author Figure 11 Culturing the C. elegans in Petri dishes Protocol Both Ampicillin and 5-Fluoro-2′-deoxyuridin will be used with NGM in the petri dishes with C. elegans http://upload.wikimedia.org/wikipedia/commons/b/b6/Ampicillin_structure.svg Figure 12 Ampicillin Figure 13 FUDR http://www.sigmaaldrich.com/structureimages/30/mfcd00006530.gif E.coli + NGM with centrophenoxine + Ampicillin + FUDR using the DAPI and GFP filter to observe amount of lipofuscin and fluorescence present in both C. elegans groups Protocol Sutphin, George; M. Kaeberlein. “Measuring Caenorhabditis elegans Life Span on Solid Media” JOVE. 2009. Figure 14 Age synchronization of C. elegans Protocol Picture by author Figure 15 Process of centrophenoxine application and observation amongst the four C. elegans groups Protocol http://upload.wikimedia.org/wikipedia/commons/7/7a/DAPI.png Figure 16 4',6-diamidino-2phenylindole (DAPI) http://www.wormbook.org/chapters/www_intromethodscellbiology/cellfig3.jpg Figure 17 C. elegans as observed under DAPI filter Budget Vendor Cat# Item Caenorhabditis Genetics Center GS1214 ham-1(ot339) C. elegans 1 $7 $7 Caenorhabditis Genetics Center AB1 Wild type C. elegans 1 $7 $7 Sigma D9542-5MG DAPI 1 $51.60 $51.60 Sigma S2002 Sodium azide 1 $21.20 $21.20 Sigma F0503-100MG FUDR 1 $117 $117 1 $97.82 $97.82 10 $6.45 $64.50 Sigma SLC5377-25G Centrophenoxine Hydrochloride Qty. Unit $ Total $ Carolina Biological 741270 Petri dishes Carolina Biological 216880 Ampicillin dry powder 1 $43.25 $43 Carolina Biological 173520 Nematode Growth Agar 2 $6.25 $12.50 Carolina Biological OP50 E. coli 1 $7 $7 Invitrogen D21490 DAPI 1 $116.00 $116.00 Total Cost $545 Do-ability Available for Purchase: The ham-1(ot339) and wild type C. elegans strains from CGC DAPI and Sodium Azide from Sigma NGM and OP50 E.coli from Carolina Biological Centrophenoxine purchaseable from Science Lab.com Equipment already Acquired: The DAPI filter (excitation filter centered at 365 nm and 445/50 nm emission band-pass filter), fluorescent microscope, UV lights Bibliography "About Parkinson Disease." National Parkinson Foundation. <”http://www.parkinson.org/Page.aspx?pid=225”>. 1996-2007. Braungart, Evelyn; Gerlach, Manfred; Riederer; Peter, Baumeister, Ralf; and Hoener, Marius C. “Caenorhabditis elegans MPP+ Model of Parkinson’s Disease for High-throughout Drug Screening.” Neurodegenerative Disease. 2004. Volume 1: pgs 175-183. Caldwin, Guy A.; K.A. Caldwell. “Traversing a wormhole to Combat Parkinson’s disease.” Disease Models and Mechanisms. Volume 1. pp.000000. 2008. Colleta, Susan. Introduction to C. elegans. Waksman Student Scholars. <http://avery.rutgers.edu/WSSP/StudentScholars/project/introduction/worms.html>. 2009 Gerstbrein, Beate; G. Stamatas; N. Kollias; M. Driscoll. “In viv spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Hall, D. H.; Z. F. Altun. “C. elegans Atlas.” Genetics Research, 90 , pp 375-376. 2008. Hunt, Sara S. The Aging Process. Washington D.C. April 2004. Kenyon, Cynthia. “Environmental Factors and Gene Activities That Influence Life Span” C. elegans II. Cold Spring Harbor Press. 1997. Kisiel, Marion J.; B. Zuckerman. “Effects of Centrophenoxine on the Nematode Caenorhabditis Briggsae” Age. Volume 1. Pp.17-20. January 1978. Mc Naught, KS; P. Jenner. “Proteasomal function is impaired in substantia nigra in Parkinson's disease “ Neuroscience Letters. Volume 297. pp. 191-194. 2001. O'Riordan ; A.M. Burnell. Intermediary metabolism in the dauer larva. II. The glyoxylate cycle and fatty acid oxidation. Comp. Biochem. Physiol. Volume 95. pp. 125-130. 1990. Rajput AH, Offord KP, Beard CM, Kurland LT. Epidemiology of parkinsonism: incidence, classification, and mortality. Ann Neurol. 1984;16:278282. Schneider, Howard F.; C. Nandy. “Effects of Centrophenoxine on Lipofuscin Formation in Neuroblastoma Cells in Culture” Journal of Gerontology. Volume 32. Pp. 132-139. 1997. Shulkin, D.J.; B.M. Zuckerman. “Spectrofluorometric analysis of the effect of centrophenoxine on lipofuscin accumulation in the nematode C. elegans.” Age. Volume 5. Pp. 50-53. 1982. Sutphin, George; M. Kaeberlein. “Measuring Caenorhabditis elegans Life Span on Solid Media” JOVE. 2009. “What is Parkinson’s?” American Parkinson Disease Association West Coast Office. <“http://www.apdawest.org/WhatIsParkinsons.html#2”>. 2009.