Hepatitis C Protease Inhibitors: Side Effects & Management
advertisement
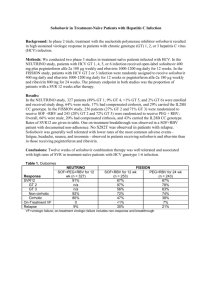
Protease Inhibitors in Chronic Hepatitis C: An Update Chapter 3 – Side Effects of Antiviral Therapy for Hepatitis C Edited by Morris Sherman MD BCh PhD FRCP(C) Associate Professor of Medicine University of Toronto November 2012 Side Effects of Antiviral Therapy for Hepatitis C Dr. Mark Levstik, FRCP(C) Associate Professor Medicine Division of Gastroenterology Multiorgan Transplant Unit London Health Sciences Centre Side Effects with Boceprevir and Telaprevir Hematological: (common to both PIs) Anemia, Neutropenia Effect is additive with INF and RBV Gastrointestinal Dysgeusia (BOC) Diarrhea (TVR & ? BOC) Anorectal irritation (TVR) Dermatological Telaprevir specific rash Side Effect Comparison of Phase III studies Adverse Effect Peg Interferon/ RBV Boceprevir/ P/R Anemia <100g/dl 30% 50% 17% 36% Rash 19% 17% 34% 56% Fatigue 59% 58% 50% 56% Diarrhoea 15% 20% 17% 26% Nausea 42% 46% 28% 39% Dysgeusia 16% 35% 3% 10% 7% 29% Anorectal Peg Interferon/ Telaprevir/ RBV P/R Dysgeusia and anemia increased with boceprevir; Rash, anorectal irritation and anemia increased with telaprevir. Incivek Product Monograph, June 2012 Victrelis Product Monograph, August 2012 Safety of Protease Inhibitors in Real Life: CUPIC Study Patients HCV genotype 1 infection Compensated cirrhosis (Child Pugh A) Treatment-experienced Relapsers Partial responders ( >2 log10 HCV RNA decline at Week 12 but never negative) Null responders theoretically excluded Treated in the French early access program (From February 2011) Hezode C et al. EASL 2012, Abstract 8 CUPIC: Treatment Regimen Interim analysis Peg-IFN + RBV BOC + Peg-IFN α-2b + RBV Follow-up BOC : 800 mg/8h; peg-IFNα-2b : 1,5 µg/kg/week; RBV : 800 -1400 mg/d TVR + Peg-IFN α-2a + RBV Peg-IFN α-2a + RBV Follow-up TVR : 750 mg/8h; peg-IFNα-2a : 180 µg/week; RBV : 1000- 1200 mg/d 0 4 8 12 16 36 Weeks 48 72 SVR assessment Hezode C et al. EASL 2012, Abstract 8 CUPIC: Patients Characteristics Baseline patient characteristics similar between BOC and TVR The CUPIC cohort had more advanced liver disease than in registration trials. In BOC arm 26% would not meet RESPOND-2 inclusion criteria In TVR arm 34% would not meet REALIZE inclusion criteria Previous treatment response (%) BOC TVR Partial responders 49 52 Relapsers 48 40 3 8 Null responders Hezode C et al. EASL 2012, Abstract 8 CUPIC: Preliminary Safety Findings (16-Week Interim Analysis) Patients, n (% patients with ≥ 1 event) Boceprevir n=159 Telaprevir n=296 Serious adverse events (%) 38.4 48.6 Premature discontinuation Due to SAEs (%) 23.9 7.4 26.0 14.5 Death (%) 1.3 2.0 Infection (Grade 3/4) (%) 2.5 8.8 0 0 6.8 0.7 Pruritus (Grade 3/4) (%) 0.6 3.7 Hepatic decompensation (%) 4.4 4.4 Rash Grade 3 (%) Grade 4 (SCAR) (%) Hezode C et al. EASL 2012, Abstract 8 CUPIC: Preliminary Safety Findings (16-Week Interim Analysis) Patients, n (% patients with ≥ 1 event) Anemia (%) Grade 2 (8.0 – <10.0 g/dL) Grade 3/4 (<8,0 g/dL) EPO use Blood transfusion Neutropenia (%) Grade 3 (500 – <1000/mm3) Grade 4 (<500/mm3) G-CSF use Thrombopenia (%) Grade 3 (25 000 – <50 000) Grade 4 (<25 000) Thrombopoïetin use Boceprevir (n=159) Telaprevir (n=296) 22.6 10.1 66.0 10.7 19.6 10.1 56.8 15.2 4.4 0.6 3.8 4.0 0.7 2.4 6.3 0.6 1.9 11.8 1.3 1.7 Hezode C et al. EASL 2012, Abstract 8 Take Home Message from CUPIC PI therapy in patients with cirrhosis is associated with more severe and more frequent AEs Anemia Increased EPO use, ribavirin dose reductions and transfusions Increased risk of severe infection Increased risk of hepatic decompensation Boceprevir Specific Side Effects Dysgeusia and decreased appetite more prevalent than control Hematological side effects more prevalent than control in Phase 2/3 naïve studies: Neutropenia (<0.75 x 109 /L): 31% vs. 18% in controls Platelets (< 50 x 109 /L): 3% vs. 1 % in controls Anemia: 50% vs. 30% in controls Grade II (<100 g/L): 49% vs. 29% Grade III (<85 g/L) : 6% vs. 3% Erythropoietin use 47% vs. 24% and pRBC 3% vs. 1% Victrelis Product Monograph, August 2012 Telaprevir Specific Side Effects Rash, anorectal disorders, diarrhea and anemia more common than control Rash seen > 50%, leads to 6% discontinuations Mild – 37% Moderate – 14% Severe – 5% Anorectal disorders seen with increase in diarrhea, itching and burning: 29% vs. 7% in controls Anemia: 32% vs. 15% in controls Grade II (<9.0-9.9 g/dL): 27% vs. 27% Grade III (7.0-8.9 g/dL) : 51% vs. 24% Incivek Product Monograph, June 2012 Anemia Management Mechanism of RBV-Associated Anemia RBV uptake into RBC adenosine kinase RBV-triphosphate Erythrocytes lack enzymes to hydrolyze RBV phosphates RBV-phosphates are “trapped” Erythrocyte T1/2 > 40 days RBV concentration in RBC 60-fold higher than serum (60:1) Marked depletion of RBC adenosine triphosphate (ATP) Impairs anti-oxidant defense mechanisms Induces RBC oxidative membrane damage Premature extravascular RBC removal by the reticuloendothelial system De Franceschi L. Hepatology 2000; 31:997-1004 Ribavirin Dose Reduction vs. EPO ? Retrospective analyses of Boceprevir phase III studies have suggested that reducing the dose of RBV did not alter the SVR rate. In patients treated with PEG+RBV (dual therapy), the effect of RBV dose reduction ON SVR was minimal if occurring when HCV-RNA was undetectable. Sulkowski MS et al. J Hepatol 2011; 54:S194-5. Reddy KR et al. Clin Gastroenterol Hepatol 2007; 5:124-9 Boceprevir Anemia Management: Erythropoietin vs. Ribavirin Dose Reduction Study Genotype 1 patients, naive of treatment, Hb < 150 g/L at baseline 687 patients treated with boceprevir RGT After completion of 4 week PEG-IFN/RBV lead-in, all patients initiated boceprevir Hemoglobin ≤100 g/L Randomisation Erythropoietin (40,000 IU/wk SC) n = 251 EPO: erythropoietin PEG-IFN: peginterferon RBV: ribavirin Ribavirin dose reduction (DR) n = 249 Hemoglobin ≤ 85 g/L: Secondary Strategy (EPO, RBV DR, transfusion) Poordad et al. EASL 2012, Abstract 1419 Results – Primary and Key Efficacy End Points End-of-treatment response, relapse, and SVR were comparable between RBV DR and EPO arms (95% CI) 100 82 82 RBV DR 71 75 Patients (%) -0.7% (-8.6, 7.2)* 71 EPO 50 25 203/249 205/251 178/249 178/251 10 10 19/196 19/197 0 EOT Response SVR Relapse DR, dose reduction; EOT, end of treatment; EPO, erythropoietin; RBV, ribavirin; SVR, sustained virologic response. *The stratum-adjusted difference (EPO vs. RBV DR) in SVR rates, adjusted for stratification factors and protocol cohort. Poordad et al. EASL 2012, Abstract 1419 Summary - Anemia Management Ribavirin dose reduction does not decrease SVR No advantage to Erythropoietin use, but may be used Consider pRBC transfusion to maintain safe Hb DAA should not be reduced DAA should not be restarted or continued without Peg/RBV Ribavirin may be increased once Hb recovers Protease Inhibitors: Management of Anemia Hb < 100 g/L any time during treatment Boceprevir Telaprevir RBV dose reduction Up to 3 x 200 mg increments* Reduce RBV to 600 mg/day Hb < 85 g/L Hb > 85 g/L EPO: 40-60,000 IU/wk AND/OR Transfusion Maintain RBV dose reduction * Note: First dose reduction of 400mg if patient receiving 1400mg/day RBV dose reduction to 600 mg can be used with Boceprevir as wel Rash Management - Telaprevir Rash Rash more prevalent in DAA but >50% with Telaprevir Rash can be categorized: Mild to moderate: < 30% of skin area Moderate: 30-50% of skin area Severe: generalized rash may progress with bullae, vesicles < 5% of patients Incivek Product Monograph, 2012 Rash Management Recommendations Mild: Watchful monitoring Oral antihistamines, moisturizers, topical steroids Moderate: < 50% body Monitor closely for progression/systemic symptoms Antihistamines, moisturizers, topical steroids Worsening/Severe: > 50% body ( < 4% of patients ) Stop telaprevir, observe closely for 7 days IF no better, stop Ribavirin, observe for 7 days. IF no better, stop Pegylated Interferon Incivek Product Monograph, 2012 Hézode C. Liver International. 2012; 32 Suppl 1:32-8 Cacoub P et al. Journal of Hepatology. 2012; 56(2):455-63 Telaprevir Severe Rash < 1% DRESS: Drug rash with eosinophilia and systemic symptoms Rash, fever, facial edema ± hepatitis/nephritis Eosinophils may not be present Stevenson-Johnson Syndrome Fever, target lesions and mucosal erosions/ulcers STOP ALL drugs Requires hospitalization May require systemic steroids Incivek Product Monograph, 2012 Hézode C. Liver International. 2012; 32 Suppl 1:32-8 Cacoub P et al. Journal of Hepatology. 2012; 56(2):455-63 Other Side Effects of Boceprevir and Telaprevir Gastroenterological Side Effects Nausea, vomiting, diarrhea Small meals three times daily with PI dosing useful Fiber, loperamide aid with loose stool Dysgeusia noted in Boceprevir patients Metallic taste, rarely leads to dose reduction or discontinuation Improved with chocolate administration Gastroenterological Side Effects: Telaprevir Nausea, vomiting and diarrhea common with TPV/PEG/RBV Anorectal irritation: Anorectal burning, itch and hemorrhoidal irritation common: > 29% Therapy: Frequent small meals, 21g fat per dose Fiber, loperamide and topical hydrocortisone therapy, help relieve symptoms Incivek Product Monograph, 2012 Hézode C. Liver International. 2012; 32 Suppl 1:32-8 Cacoub P et al. Journal of Hepatology. 2012; 56(2):455-63 Management of Depression Occurs in up to 37% of patients Conduct pre-therapy and routine assessments with CES-D or other depression scale Adjust interferon dose or discontinue therapy according to depression severity May warrant use of antidepressants Recommended agents to use with BOC and TVR: Escitalopram, citalopram (see Dr. Tseng’s chapter on DDIs) Direct-Acting Antiviral Therapy: Boceprevir and Telaprevir Patient side-effect education is important to success Pre-therapy recommendations include: Multivitamin, hydration, acetaminophen analgesia Dietary recommendations to decrease GI toxicity effects ( small meals, fiber, loperamide ) Skin care through moisturizers and antihistamines Close patient and hepatitis team communication Monitor and pre-empt severe side effects Drug and duration specific The Canadian Liver Foundation gratefully acknowledges the participating health care professionals for their contributions to this project and for their commitment to the liver health of Canadians. The Canadian Liver Foundation (CLF) was the first organization in the world devoted to providing support for research and education into the causes, diagnoses, prevention and treatment of all liver disease. Through its chapters across the country, the CLF strives to promote liver health, improve public awareness and understanding of liver disease, raise funds for research and provide support to individuals affected by liver disease. For more information visit www.liver.ca or call 1-800-563-5483. This project made possible through the financial support of Merck Canada Inc. The views, information and opinions contained herein are those of the authors and do not necessarily reflect the views and opinions of Merck Canada Inc.