PROTEOME:
advertisement

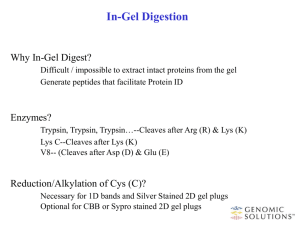
PROTEOMICS LECTURE The “omics” nomenclature… Genomics DNA (Gene) Transcription Transcriptomics RNA Translation Functional Genomics Proteomics PROTEIN Enzymatic reaction Metabolomics METABOLITE A few definitions… Gen Transcript Prote Metabol Gen Prote ~ome = Sequence of a complete set of ~omics = Analysis of the Genes Transcripts Proteins Metabolites Genome Proteome Why study protein expression? (The steps of gene expression control) (Gygi et al., Mol. Celll. Biol., 1990, p.1720-1730) Nucleus Cytosol Inactive mRNA RNA Degradation control DNA Primary RNA transcript mRNA mRNA Translation control Transcriptional control RNA Processing control RNA Transport control protein Modified protein Post-translational control Applications of Proteomics • Mining: identification of proteins (catalog the proteins) • Protein-expression profile: identification of proteins in a particular state of the organism • Protein-network mapping: protein interactions in living systems • Mapping of protein modifications: how and where proteins are modified. Proteins classes for Analysis • • • • • • • Membrane Soluble proteins Nuclear Chromosome-associated Phosphorylated Glycosylated Complexes SEPARATION IDENTIFICATION General flow for proteomics analysis Current Proteomics Technologies • Proteome profiling/separation – 2D SDS PAGE (two-dimensional sodium dodecylsulphate polyacrylamide gel electrophoresis) – 2-D LC/LC (LC = Liquid Chromatography) – 2-D LC/MS (MS= Mass spectrometry) • Protein identification – Peptide mass fingerprint – Tandem Mass Spectrometry (MS/MS) • Quantative proteomics - ICAT (isotope-coded affinity tag) 2D-SDS PAGE gel 1) Sample loading 3)Place the strip gel in the focusing tray 2) Remove the cover sheet from the IEF gel 4) Place the strip on the top of the SDS-PAGE gel 2D-SDS PAGE gel The first dimension (separation by isoelectric focusing) - gel with an immobilised pH gradient - electric current causes charged proteins to move until it reaches the isoelectric point (pH gradient makes the net charge 0) Isoelectric point (pI) • Separation by charge: 4 Stable pH gradient 5 Low pH: Protein is positively charged 6 7 8 9 10 High pH: protein is negatively charged At the isolectric point the protein has no net charge and therefore no longer migrates in the electric field. 2D-SDS PAGE gel The first dimension (separation by isoelectric focusing) - gel with an immobilised pH gradient - electric current causes charged proteins to move until it reaches the isoelectric point (pH gradient makes the net charge 0) The second dimension (separation by mass) -pH gel strip is loaded onto a SDS gel -SDS denatures and linearises the protein (to make movement solely dependent on mass, not shape) 2D-SDS PAGE gel 2D-gel technique example Advantages vs. Disadvantages • Good resolution of proteins • Detection of posttranslational modifications • Not for hydrophobic proteins • Limited by pH range • Not easy for low abundant proteins • Analysis and quantification are difficult 2D - LC/LC Study protein complexes without gel electrophoresis Complex mixture is simplified prior to MS/MS by 2D LC (trypsin) Peptides all bind to cation exchange column Successive elution with increasing salt gradients separates peptides by charge Peptides are separated by hydrophobicity on reverse phase column Reverse Phase column Polypeptides enter the column in the mobile phase… …the hydrophobic “foot” of the polypeptides adsorb to the hydrophobic (non polar) surface of the reverse-phase material (stationary phase) where they remain until… …the organic modifier concentration rises to critical concentration and desorbs the polypeptides 2D - LC/MS Methods for protein identification Mass Spectrometry (MS) Stages • Introduce sample to the instrument • Generate ions in the gas phase • Separate ions on the basis of differences in m/z with a mass analyzer • Detect ions How the protein sequencing works? • Use Tandem MS: two mass analyzer in series with a collision cell in between • Collision cell: a region where the ions collide with a gas (He, Ne, Ar) resulting in fragmentation of the ion • Fragmentation of the peptides in the collision cell occur in a predictable fashion, mainly at the peptide bonds (also phosphoester bonds) • The resulting daughter ions have masses that are consistent with known molecular weights of dipeptides, tripeptides, tetrapeptides… Ser-Glu-Leu-Ile-Arg-Trp Collision Cell Ser-Glu-Leu-Ile-Arg Ser-Glu-Leu-Ile Ser-Glu-Leu Etc… Tandem Mass Spectrometry (trypsin) Isolates individual peptide fragments for 2nd mass spec – can obtain peptide sequence Compare peptide sequence with protein databases Advantages vs. Disadvantages • Determination of MW and aa. Sequence • Detection of posttranslational modifications • High-throughput capability • High capital costs • Requires sequence databases for analysis Protein identification by Peptide Mass fingerprint • Use MS to measure the masses of proteolytic peptide fragments. • Identification is done by matching the measured peptide masses to corresponding peptide masses from protein or nucleotide sequence databases. Mass spectometry (MS) (trypsin) eg. MALDI-TOF Mass spectrometry – method of separating molecules based on mass/charge ratio Compare peptide m/z with protein databases Protein Identification by MS Library Spot removed from gel Artificial spectra built Fragmented using trypsin Spectrum of fragments generated MATCH Artificially trypsinated Database of sequences (i.e. SwissProt) ISOTOPE-CODED AFFINITY TAG (ICAT): a quantitative method • Label protein samples with heavy and light reagent • Reagent contains affinity tag and heavy or light isotopes Chemically reactive group: forms a covalent bond to the protein or peptide Isotope-labeled linker: heavy or light, depending on which isotope is used Affinity tag: enables the protein or peptide bearing an ICAT to be isolated by affinity chromatography in a single step Example of an ICAT Reagent Biotin Affinity tag: Binds tightly to streptavidin-agarose resin Reactive group: Thiolreactive group will bind to Cys O Linker: Heavy version will have deuteriums at * Light version will have hydrogens at * NH NH H N * S O * O O * O * H N I O How ICAT works? Affinity isolation on streptavidin beads Lyse & Label Quantification MS NH2-EACDPLR-COOH Light 100 Identification MS/MS 100 MIX Heavy Proteolysis (eg trypsin) 0 550 570 m/z 590 0 200 400 m/z 600 Advantages vs. Disadvantages • Estimates relative protein levels between samples with a reasonable level of accuracy (within 10%) • Can be used on complex mixtures of proteins • Cys-specific label reduces sample complexity • Peptides can be sequenced directly if tandem MS-MS is used • Yield and non specificity • Slight chromatography differences • Expensive • Tag fragmentation • Meaning of relative quantification information • No presence of cysteine residues or not accessible by ICAT reagent