Unit 2 - Belle Vernon Area School District
advertisement

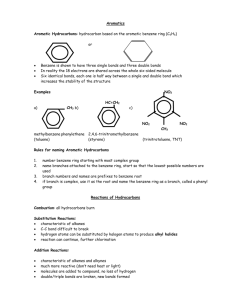
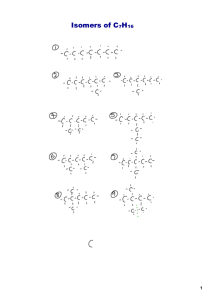
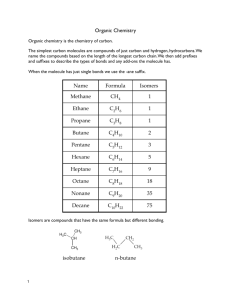
Unit 2 Introduction to Hydrocarbons Differences between organic and inorganic compounds: 1. Organic compounds are mostly covalent molecules where most inorganics are ionic 2. Most organics don’t dissolve in water and most inorganics do 3. Organic compounds decompose on heating easier than inorganics 4. Organic reactions are much slower (min, hours, days) than inorganic reactions (seconds) Fun Facts (Don’t have to Copy) More than 18 Million organic compounds [with 10,000 new ones discovered each year] 1.7 Million inorganic compounds so about 85% of compounds are organic 2 Reasons for the abundance of organic compounds: Carbon atoms bond to each other to form long chains(up to 200 carbons) Catenation – the ability of an element to bond to itself The same number of carbon atoms can rearrange to form different structures (isomers) Isomer – compounds with the same molecular formula but different structures Isomer Example C5H12 C -C-C-C-C-C- -C-C-C-C- C -C-C-CC How Carbon Bonds C ground state is 2s22p2 but bonds as *2s12p3 giving 4 sp3 hybrid orbitals Hybrid orbitals – orbitals of equal energy formed by mixing orbitals of different energies Hybridization – the mixing of orbitals of different energies to give orbitals of equal energy How Carbon Bonds Carbon’s 4 covalent bonds form a tetrahedron (109.5° bond angle) Hydrocarbons! Hydrocarbons – compounds containing only hydrogen and carbon Alkanes – hydrocarbons that have all C-C single bonds Naming # of C Name 1 Structural Formula Methane -C- Condensed Formula CH4 Molecular Formula CH4 2 Ethane CH3CH3 CH3CH2 CH3 CH3(CH2)2 CH3 CH3(CH2)3 CH3 CH3(CH2)4 CH3 CH3(CH2)5 CH3 CH3(CH2)6 CH3 C2H 6 C3H 8 C4H10 C5H12 C6H14 C7H16 C8H18 CH3(CH2)7 CH3 C9H20 CH3(CH2)8 CH3 C10H22 3 4 5 6 7 8 9 10 -C-CPropane -C-C-CButane -C-C-C-CPentane -C-C-C-C-CHexane -C-C-C-C-C-CHeptane -C-C-C-C-C-C-COctane -C-C-C-C-C-C-CCNonane -C-C-C-C-C-C-CC-CDecane -C-C-C-C-C-C-CC-C-C- Naming *Know roots and endings* Each step, you add a CH2 group Homologous Series – a series of compounds where each member differs from the next by a constant unit (CH2) Members are called homologous Since alkanes are homologous – we can write a General Formula = CnH2n+2 Naming Alkanes are Saturated Hydrocarbons – hydrocarbons where each C has 4 single covalent bonds (no more atoms can be added) Alkenes Alkenes – hydrocarbons with one C=C double bond – sp2 hybridization on the 2 C atoms in the double bond. Ethene C=C CH2CH2 C2H4 Propene C=C-C CH2CHCH3 C3H6 Butene C=C-C-C CH2CHCH2CH3 C4H8 Octene C=C-C-C-C-C-C-C- CH2CH(CH2)5CH3 C8H16 Ways to Show Organics Line Bond Form Space Filling Model Ball and Stick Form Structural Formula Skeletal Form Alkenes Also a homologous series General Formula CnH2n Unsaturated hydrocarbons – have C-C multiple bonds which can be broken to add more atoms to the molecule H Ex: H C=C H H H + H2 H H-C -C-H H H Alkynes Alkynes – hydrocarbons containing a C = C triple bond – sp hybtidization Ethyne -C=C- CHCH C2H2 (acetylene) Propyne -C=C-C- CHCCH3 C 3 H4 Butyne -C=C-C-C- CHCCH2CH3 C 4 H6 Heptyne -C=C-C-C-C-C-C- CHC(CH2)4CH3 General Formula = CnH2n-2 C7H12 Alkadienes Alkadienes – hydrocarbons containing two C=C double bonds Butadiene -C=C-C=C- Pentadiene -C=C-C=C-C- CH2(CH)2CH2 C4H6 CH2(CH)3CH3 C5H8 Heptadiene -C=C-C=C-C-C-CCH2(CH)3(CH2)2CH3 General Formula = CnH2n-2 C7H12 Alkadienes 3 placements for the two double bonds Conjugated double bonds (most common) – two double bonds separated by one singe bond Isolated double bonds – two double bonds separated by more than one single bond Allenes – hydrocarbons that have two consecutive double bonds The first 4 Series of hydrocarbons are Aliphatic Hydrocarbons Aliphatic hydrocarbons – hydrocarbon where carbon atoms bond together in open chains Arenes Aromatic Hydrocarbons – hydrocarbons containing rings of 6 carbon atoms joined by alternating single and double bonds Simplest aromatic hydrocarbon = benzene Arenes All bonds are actually identical (C-C and C=C “mixed”) Can also be shown as Arenes We use The e-‘s are actually shared by all 6 carbons and move freely around the ring (delocalized) This makes benzene behave like saturated hydrocarbons Resonance Compounds like these are resonance hybrids (compounds that can be represented by more than one Lewis structure) General Formula = CnH1/2n+3 Resonance examples IUPAC Naming Rules 1. Name the longest chain (the parent chain) first. 2. Label the chain to give the lowest numbers to groups or bonds. Priority C=C then C=C You give the number for the carbon where the multiple bond begins. (Separate numbers and words with a hyphen, and numbers and numbers with a comma). 6 5 4 3 2 1 C-C-C-C=C-C 2 –hexene C-C-C=C-C-C-C 3 – heptyne IUPAC Naming Rules 3. Give the numbers for any attached groups for the carbons they are attached to, a number for each attached group. Use the number with the groups name. [in front of “main” chain] a) If more than one of any group = di-, tri-, tetra-, penta-, hexa-, etc. b) Group Names: F = fluoro I = iodo Cl = chloro OH = hydroxo Br = bromo NO2 = nitro IUPAC Naming Rules c. If there is more than one group attached, the names are listed in alphabetical order (ignore prefixes) in front of the “main” chain d. If the numbers for the side groups are the same from either side of the chain, # from the side that gives the lowest # to the first group in the alpha order. Summary Hydrocarbons (straight chains) Locate and name attached groups Locate multiple bonds (priority for numbering) Name base/parent chain IUPAC Naming Rules 4. Branched chains Longest continuous chain containing any multiple bonds (if present) # to give multiple bonds lowest numbers (priority) Name side groups (alphabetical order) IUPAC Naming Rules 1-chloro-3,5 - dimethylbezene 5. Benzene Number starting with a C bonded to an attached group and then continue around the ring Use the lowest set of #’s possible IUPAC Naming Rules 1,3 – dibromo – 2 – fluorobenzene IUPAC Naming Rules c. If there are just two of the same group attached, we can use the following terms to simplify the bonding positions Ortho = 1, 2 bonding position Meta = 1, 3 bonding position Para = 1, 4 bonding position O,M, P Isomer Practice Isomers – compounds having the same molecular formula but having different structures Example: C5H11Cl Draw all isomers by moving Cl (we are only going to use straight chains for C’s) Isomer Practice Cl C–C–C–C–C Cl C –C – C – C – C Cl C–C–C–C–C Isomer Practice C4H8Cl2 Isomer Practice Try C4H8ClI, C4H7I3, C5H10FBr, and C4H7F2Br