IgG and IgM based immunopathological reaction (reaction of
advertisement
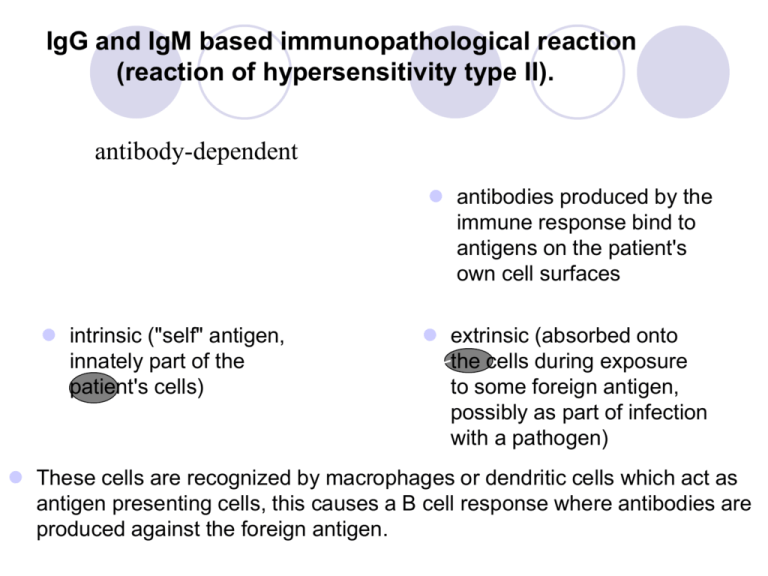
IgG and IgM based immunopathological reaction (reaction of hypersensitivity type II). •= antibody-dependent antibodies produced by the immune response bind to antigens on the patient's own cell surfaces intrinsic ("self" antigen, innately part of the patient's cells) extrinsic (absorbed onto the cells during exposure to some foreign antigen, possibly as part of infection with a pathogen) These cells are recognized by macrophages or dendritic cells which act as antigen presenting cells, this causes a B cell response where antibodies are produced against the foreign antigen. IgG and IgM based immunopathological reaction (reaction of hypersensitivity type II) Autoimmune hemolytic anemia Goodpasture's syndrome Autoimmune pernicious anemia Immune thrombocytopenia Transfusion reactions Myasthenia gravis Rheumatic fever Acute transplant rejection Immune complex based immunopathological reaction (reaction of hypersensitivity type III) occurs when antigens and antibodies are present in roughly equal amounts, causing extensive cross-linking large immune complexes that cannot be cleared are deposited in vessel walls and induce an inflammatory response the reaction can take hours, days, or even weeks to develop Immune complex based immunopathological reaction (reaction of hypersensitivity type III) Some clinical examples: Rheumatoid arthritis Immune complex glomerulonephritis Serum sickness Subacute bacterial endocarditis Systemic lupus erythematosus Farmer's lung (Arthus-type reaction) Polyarteritis nodosa PRIMARY IMMUNODEFICIENCY clinical manifestactions examples IMMUNODEFICIENCY Primary immunodeficiencies - congenital, genetically defined disorders - onset of symptoms - predominantly at an early age Secondary immunodeficiencies - caused by chronic infections, irradiation, injuries, immunosupression therapy, surgery, stress - disorders appear at any age IMMUNIDEFICIENCY Humoral deficiency disorders = the B cell deficiency disorders – the qualitative or quantitative defects of the B cells, present 70% of IDs T cell deficiency disorders and the combined B-cell and T-cell deficiency disorders (20%) – group of the qualitative or quantitative defects of the T and B cells Phagocytic cell disorders– group of the qualitative or quantitative defects of the fagocytic cells (10%) Complement disorders – caused by the deficiency of the complement components or functions (<1%) MAJOR CLINICAL FEATURES Humoral deficiency disorders - manifest as the recurrent bacterial sinopulmonary and gastrointestinal infections - caused by streptococcus, staphylococcus, haemophilus, begin when infants are 5-9 months of age T cell disorders - manifest as the recurrent bacterial, fungal and viral respiratory and gastrointestinal infection Complement disorders – are associated with increased incidence of the infections and autoimmune diseases and with edema in the case of hereditary angioedema Phagocytic cell disorders – characterized by recurrent infections caused by various organisms incluging abscesses, purulent skin infections, granulomatous inflammations HUMORAL DEFICIENCY DISORDERS Bruton’s X-linked hypogamaglobulinemia CVID - Common Variable ImmunoDeficiency Selective immunoglobulin A deficiency <0,07 g/l Bruton’s X-linked hypogamaglobulinemia the genetic defect on the X chromosome leads to the defective function of a tyrosine kinase in the B cells This defect result in a block of the pre-B cells maturation into the B cells with surface IgM the immunologic findings: < 2% circulating B cells - low serum levels of all classes of immunoglobulins - number and function of T cells are intact - pre-B cells are in the bone marrow features : begining from 5-9 months of age - manifests as recurrent bacterial sinopulmonary and gastrointestinal infection caused by streptococcus, staphylococcus, haemophilus, meningococcus, salmonella, campylobacter, giardia Treatment consists of life-long intravenous pooled human gammaglobulin replacement and antibiotics. Common Variable ImmunoDeficiency the B cell functional disorder characterized by the normal number of the B cells, low levels of IgG and IgA, a poor response to all vaccines and decrease of the T cells (CD4+) number and function the symptom’s onset between 2nd and 3rd decade the clinical features: - recurrent respiratory tract infections (pneumonia), cutaneous and gastrointestinal infection - disease is accompanied by occurrence of the granulomas, lymphadenopathy, splenomegaly Treatment consist of the intramuscular or intravenous gammaglobulin replacement. Selective deficiency of IgA level of IgA up to 0,05 g/l, age > 4 years the most frequent primary ID - stem cell defect - repeated infections of respiratory tract - susceptibility to autoimmune disorders, malignant disorders, allergy - contra-indication of administration of drug with IgA T cell deficiency disorders DiGeorge syndrome - the genetic defect on the chromosome 22 leads to disorder of development of 3rd and 4th branchial pouch with congenital hypoplasia of both the thymus and parathyroid glands - patients suffer from disorder of pre-thymocytes maturation due to absence/hypoplasia of thymus - syndrome CATCH 22: cardiac defects, abnormal facies, thymic hypo/aplasia, cleft palate, hypocalcemia, deletion 22q11.2 - the symptom’s onset soon after the birth – hypocalcemic spasms and manifestations of congenital heart disease - treatment: symptomatic, transplantation of a thymus PRIMARY FAGOCYTIC CELL DEFECTS Chronic granulomatous disease - X- linked recesive disorder - leads to defect in neutrophilic cytochrome b with suppresion of intracellular killing of ingested microorganisms - normal number of leucocytes - infection of catalase-positive bacterias - symptoms appear in the first year of age: pyogenic cutaneous infections, abscesses, granulomas in many organs, pyogenic lymphadenitis - treatment: long-term ATB administration, interferon gamma, corticosteroids COMPLEMENT DEFICIENCY C2, C3, C4 complement components deficiencies - lead to an impaired opsonization, susceptibility to infections, autoimune diseases C6, C7, C8, C9 complement components deficiencies - lead to the autoimmune diseases – SLE, RA, sclerodermia and to the neisserial infection MBL deficiencies - lead to the respiratory infections and susceptibility to the autoimune and allergy diseases Treatment: vaccination, ATB HEREDITARY ANGIOEDEMA pathophysiology clinical manifestations treatment HEREDITARY ANGIOEDEMA the congenital AD complement disorder cased by the defect on the chromosome 11 leads to absence or functional deficiency of C1-inhibitor C4 a C2 complement components show a low level during atack Type I - occurs in 85% - an absence of C1-inhibitor Type II - occurs in 15% - a functional deficiency of C1-inhibitor Secondary - SLE, lymfoma HEREDITARY ANGIOEDEMA C1 esterase inhibitor deficiency leads to uncontrolled C1 activity and resultant production of a kinin that increases capillary permeability Clinical feature: transient recurrent localized edema the triggering factors: injuries or surgical/stomatological operations more offen occures in pregnancy laryngeal edema could be life-threatening, immediate treatment is necessary ! TREATMENT Preventive – consist of an administration of androgens, a-fibrinolytics - before operation is necessary C1-INH concentrate or a fresh frozen plasma administration - stomatology procedures are performed in hospital Immediate - C1-INH concentrate or fresh frozen plasma administration tracheotomy in severe larynx edema treatment with ACE inhibitors is contraindicated ACQUIRED IMMUNODEFICIENCIES causes mechanisms involved AIDS ACQUIRED IMMUNODEFICIENCIES Acute and chronic viral infections – EBV, CMV, herpetic virus, influenza, HIV Metabolic disorders – diabetes, renal failure, disorder of liver function Autoimmune diseases – autoantibodies against immunocompetent cells (neutrophils, lymphocytes) Allergic diseases Chronic GIT diseases, nephrotic syndrome Malignant diseases (leukemia, lymphoma, myeloma) Hypersplenism/asplenia, splenectomy – deficiency in generation of antibodies against encapsulated microorganisms (Pneumococcus, Neisseria) Burn, postoperative status, injuries Severe nutritional disorders, chronic stress Drug induced immunodeficiencies (chemotherapy), immunosupression Chronic exposure to harmful chemical substances, ionizing radiation AIDS Acquired ImmunoDeficiency Syndrom - caused by a retrovirus called human immunodeficiency virus - current incidence 40 mil.people, predominantly in central Africa, CZ – about 1000 infected people viral transmission occurs through: - sexual intercourse - contact with blood - transplacentally, during the birth process or through a breast milk VIRUS HIV-1 virion is consisted of a capside with marrow protein - p24 and RNA RNA is copied into double-stranded DNA using reverse transcriptase virus integrates to the human cell genome and arise a provirus an activation of provirus leads to the replication of viral nuclear acid and genesis of a virion that goes through the cell membrane and caused the lysis of cell PRIMARY INFECTION Infection - begins by HIV-1 with a tropism for macrofages: - the membrane molecules of dendritic cells bind glycoproteins on HIV-1 surface and transport viruses to the lymphatic nodes (LN), where activated T cells are infected viruses are replicated in the lymphatic nodes and transfer to the blood features: malaise, fever, pain of muscles and joints, sweating, loss of appetite, vomiting, diarrhoea, rash, lymphadenopathy Immunological findings: elevated C-reactive protein, lymphopenia, decrease of CD4+ cells specific antibodies against HIV-1 don‘t generate identification of viruses is performed by PCR or by the evidence of viral protein p24 presence ASYMPTOMATIC PERIODE asymptomatic period – HIVs-1 with a tropism for macrophages are changed into viruses with a tropism for T cells and demage T cells (CD4+) viruses replicate in cell secondary lymphatic organs - the period can last a several years lasting depends on: - virus doses and virulence - an individual condition of immune system an infected person - an acceleration occures by repeated infection of different HIVs AIDS AIDS- Related Complex (ARC) presents with lymphadenopathy and comes before fully developed AIDS Clinical features of AIDS : - candidiasis of mouth and esophagous mucose, colpitis - oral leucoplakia, opportunistic infections - Kaposi sarcoma, non-Hodgkin‘s lymfoma VACCINE development of a vaccine is unsuccessful due to: - unsuccesful searching for a dominant viral antigen - variability of the viruses HIV-1 in the course of time - absence of an animal experimental model (even the primate‘s infection course isn‘t identical with human) TREATMENT Inhibitors of reverse transcriptase - 2 types + Inhibitor of viral protease = Therapy result to the inhibition of DNA synthesis, stop the progress of the disease and prolong the life of HIV infected persons IMMUNOGLOBULIN REPLACEMENT THERAPY Indication Contra-indication Adverse reaction IVIG is approved for treating X-linked Bruton agammaglobulinemia Common Variable ImmunoDeficiency others CONTRA-INDICATIONS Repeated severe side effects Selective IgA deficiency with anaphylactic reaction to immunoglobuline Severe acute infection IG ADMINISTRATION Intramuscullar – maximum dose 1,5 g IgG/ week Subcutaneous – total dose/month 400mg/kg, administration every week Intravenous - 400 mg/kg/month AUTOIMMUNE DISORDERS examples CLINICAL CATEGORIES systemic - affect many organs and tissue organ localised - affect predominantly one organ accompained by affection of other organs (nonspecific bowel diseases, celiatic disease, AI hepatitis, pulmonary fibrosis) organ specific - affect one organ or group of organs connected with development or function EXAMPLES OF SYSTEMIC AUTOIMMUNE DISEASES examples autoantibodies SYSTEMIC AUTOIMMUNE DISEASES Systemic lupus erythematosus Rheumathoid arthritis Sjögren‘s syndrome Dermatopolymyositis Systemic sclerosis Mixed connective tissue disease Antiphospholipid syndrome Vasculitis Sarcoidosis SYSTEMIC LUPUS ERYTHEMATOSUS chronic, inflammatory, multiorgan disorder predominantly affects young women autoantibodies react with nuclear material and attack cell function, immune complexes with dsDNA deposit in the tissue general symptoms: include malaise, fever, weight loss multiple tissue are involved including the skin, mucosa, kidney, joints, brain and cardiovascular system characteristic features: butterfly rash, renal involvement, CNS manifestation, pulmonary fibrosis DIAGNOSTIC TESTS a elevated ESR (erythrocyte sedimentation rate), low CRP, trombocytopenia, leukopenia, hemolytic anemia, depresed levels of complement (C4, C3), elevated serum gamma globulin levels AUTOANTIBODIES Autoantibodies: ANA, dsDNA (doublestranged), ENA (SS-A/Ro, SS-A/La), Sm, against histones, phospholipids RHEUMATOID ARTHRITIS chronic, inflammatory joint disease with systemic involvement predominantly affects women characterized by an inflammatory joint lesion in the synovial membrane, destruction of the cartilage and bone, results in the joint deformation clinical features: arthritis, fever, fatigue, weakness, weight loss systemic features: vasculitis, pericarditis, uveitis, nodules under skin, intersticial pulmonary fibrosis diagnostic tests: elevated C- reactive protein and ESR, elevated serum gammaglobulin levels - autoantibodies against IgG = rheumatoid factor (RF), a-CCP (cyclic citrulline peptid), ANA - X-rays of hands and legs- show a periarticular porosis, marginal erosion Antiphospholipid syndrome autoimmune disease characterized by vein and arterial thrombosis, repeated abortions accompanied by anti-phospholipid autoantibodies (APA) and antibodies against β2-glykoprotein I EXAMPLES OF ORGAN- SPECIFIC AUTOIMMUNE DISEASES diseases autoantibodies ORGANOLEPTIC AUTOIMMUNE DISEASES Ulcerative colitis Crohn‘s disease Coeliac disease Autoimmune hepatitis Primary biliary cirhosis Primary sclerotic cholangoitis Pulmonary fibrosis Ulcerative colitis chronic inflammation of the large intestine mucose and submucose features: diarrhea mixed with blood and mucus extraintestinal features (artritis, uveitis) autoantibodies against pANCA, a- large intestine Crohn‘s disease the granulomatous inflammation of all intestinal wall with ulceration and scarring that can result in abscess and fistula formation the inflammation of Crohn's disease the most commonly affects the terminal ileum, presents with diarrhea and is accompanied by extraintestinal features - iridocyclitis, uveitis, artritis, spondylitis antibodies against Saccharomyces cerevisiae (ASCA), a- pancreas Coeliac disease a malabsorption syndrome characterized by marked atrophy and loss of function of the villi of the jejunum inflammatory bowell disease arise from gliadin exposition autoantibodies against endomysium, the most specific = tissue transglutaminaze; antibodies against gliadin are nonspecific biopsy of the jejunum with findings of the villi atrophy ORGAN SPECIFIC AUTOIMMUNE DISEASES Autoimmune endocrinopathy Autoimmune neurological diseases Autoimmune cytopenia Autoimmune cutaneous diseases Autoimmune eye diseases AUTOIMMUNE ENDOCRINOPATHY Hashimoto‘s thyroiditis Graves-Basedow disease Postpartum thyroiditis Diabetes mellitus I. type Addison‘s disease Autoimmune polyglandular syndrome Pernicious anemia Hashimoto‘s thyroiditis thyroid disease result to hypothyroidism on the base of lymphocytes and plasma cells infiltrate autoantibodies against thyroidal peroxidase (aTPO) and/or against thyroglobulin (a-TG) Grave‘s disease thyrotoxicosis from overproduction of thyroid hormone (patient exhibit fatigue, nervousness, increased sweating, palpitations, weight loss, exophtalmos) autoantibodies against thyrotropin receptor, autoantibodies cause thyroid cells proliferation Diabetes mellitus (insulin- dependent) characterized by an inability to process sugars in the diet, due to a decrease in or total absence of insulin production results from immunologic destruction of the insuline- producing β-cells of the islets of Langerhans in the pancreas autoantibodies against GAD- glutamic acid decarboxylase = primary antigen), autoantibodies anti- islet cell, anti- insulin islets are infiltrated with B and T cells AUTOIMMUNE NEUROPATHY Guillain-Barré syndrome (acute idiopathic polyneuritis) Myasthenia gravis Multiple sclerosis Myasthenia gravis chronic disease resulting from faulty neuromuscular transmission characterized by muscle weakness and fatigue the muscle weakness and neuromuscular dysfunction result from blockage and depletion of acetylcholin receptors at the myoneural junction immunological findings: autoantibodies against Ach receptors ptosis of the eye Multiple sclerosis chronic demyeline disease with abnormal reaction T cells to myeline protein on the base of mimicry between a virus and myeline protein features: weakness, ataxia, impaired vision, urinary bladder dysfunction, paresthesias, mental abberations autoantibodies against MOG (myelin-oligodendrocyte glycoprotein) Magnetic resonance imaging of the brain and spine shows areas of demyelination The cerebrospinal fluid is tested for oligoclonal bands, can provide evidence of chronic inflammation of the central nervous system IMMUNOSUPRESSION non-specific treatment examples of drugs indication risks Immunosuppressants are drugs that inhibit or prevent activity of the immune system They are used in immunosuppressive therapy to: Prevent the rejection of transplanted organs and tissues Treat autoimmune diseases Treat some other non-autoimmune inflammatory diseases (allergic asthma, atopic eczema) Glucocorticoids suppress the cell-mediated immunity cytokine production suppress the humoral immunity side-effects: hypertension, dyslipidemia, hyperglycemia, peptic ulcers, osteoporosis, disturbed growth in children Drugs affecting the proliferation of both T cells and B cells - Cyclophosphamide, Methotrexate, Azathioprine, Mycophenolate mofetil Drugs blocking the activation of lymphocytes – Tacrolimus, Sirolimus, Cyclosporin A Monoclonal antibodies - Daclizumab