Pictures for this lecture
advertisement

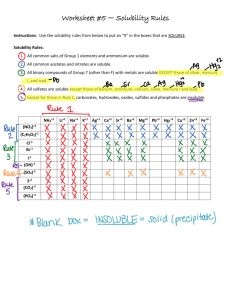
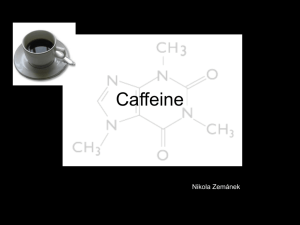
Today: • Quiz Comments • Practice for Melting Point Ranges and Recrystallization • Introduction to Molecular Modeling (Lab today is in SEGA lab Science249) Melting point ranges for mixtures of compounds A and B Phase Diagram What is the mp. and mol% composition of the eutectic mixture? What is the mp. range of mixture X? o C oC 140 Pure B Pure A X 100 80 60 60 40 E 20 20 Solid A and B mol%A 100%A 75%A 50%A 25%A mol%B 25%B 50%B 75%B 100%B The following solubility data were collected before recrystallizing a sample of caffeine (C): Solvent Sol. Of C at 20o Sol of C in hot solvent Water Ethanol Acetone Benzene Pet. Ether slightly soluble soluble soluble slightly soluble insoluble soluble soluble soluble soluble insoluble 1) Which single solvent(s) could you use for recrystallization? 2) Suggest a mixed solvent pair that you could use instead. Solubility Data for Caffeine 1.000g of caffeine completely dissolves in a minimum of – 45.0ml of water at 14oC – 5.5 ml of water at 80oC – 1.5 mL of boiling water Assume that you are recrystallizing 2.000g of impure caffeine. After one recrystallization from 30.0 ml of water you ended up with 1.200 g of purified caffeine. How many grams of caffeine were left in the cooled water? (careful …) If you reduced the volume of the mother liquor to 5 ml, how many grams of caffeine would be left in solution in the cooled solution? Molecular Modeling Remember these: Hybridization of C Bond length, angle, dihedral angle; staggered and eclipsed conformations Conformations of cyclohexane; axial , equatorial substituents We’ll do MM Exps. 1, 2, 4, only Next time: Distillation/Gas Chromatography (Exp.3) (Notebook preparation please… Preview/prestudy experiment)