document
advertisement

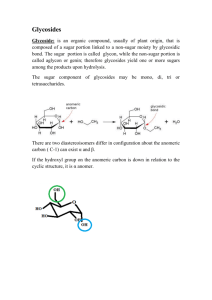
Glycosides • Definition: Organic natural compounds present in a lot of plants and some animals, these compounds upon hydrolysis give one or more sugars (glycone) β_form and non sugar (aglycone) or called genin. • Solubility: glycosides are water soluble compounds and insoluble in the organic solvents. Glycone part: water soluble, insoluble in the organic solvents. Aglycone part: water insoluble, soluble in the organic solvents. Some glycosides soluble in alcohol. Separation between glycosides parts: Glycosides Hydrolysis glycone +aglycone +HCL +HCLdil Neutralization by Filtration G + A +salt+H 2 O Using alkaline chloroform (H2O+G)+A (H2O+G)+(chloroform+A) We can separate them by using separatory funnel The best solvent to extract aglycone is Ethyl acetate because: A- immiscible in water. B- always presents in the upper layer. Note: Alcohol and acetone are water miscible compounds,so we can't use them as organic solvents for aglycone separation. physico-chemical properties of glycosides(general) • Colorless, solid, amorphous, nonvolatile (flavonoid- yellow, anthraquinone-red or orange. • Give positive reaction with Molisch's and Fehling's solution test (after hydrolysis). • They are water soluble compounds, insoluble in organic solvents • Most of them have bitter taste (except: populin, glycyrrhizin, stevioside). Cont... • Odorless except saponin (glycyrrhizin). • when a glycosides has a lot of sugars its solubility in water decrease. • Glycosides hydrolyzed by using mineral acids and temperature or by using enzymes such as: a- Emolsin Bitter almond seeds. b- Myrosin or Myrosinase black mustard seeds. c- Rhamnase glycosides containing rhamnose as sugar part. Biosynthesis of glycosides (O-glycosides) • UTP(Uridine Triphoshate) +sugar-1-phoshate UDP-sugar + ppi(Pyrophosphate inorganic). Glycosyl transferase • UDP-sugar +acceptor (aglycone) Enzyme Glycosyl transferase Acceptor-sugar +UDP Enzyme Uridylyl transferase Enzyme The function or the role of glycosides in the plant organism • Converting toxic materials to non or less toxic. • Transfer water insoluble substances by using monosaccharide. • Source of energy (sugar reservoir). • Storing harmful products such as phenol. • Regulation for certain functions(growth). • Some have beautiful colours(pollenation process). Cont… • Some glycosides have antibacterial activity, so they protect the plants from bacteria and diseases. bacteria kill Bitter almond Amygdalin Eomlsin enzyme hydrolysis HCN Classification of glycosides Classifications of glycosides according to their therapeutic effects • CHF and cardiac muscles stimulators such as: a-Digitalis glycosides: digoxin, digitoxin, gitoxin (Fox glove leaves). b- Ouabain: Strophanthus gratus seeds. c- K-strophanthin -Strophanthus kombe seeds. d- Scillaren A,B which isolated from red and white Squill bulbs. e- Convolloside:Convallaria majalis – Lily of the Valley. Cont… • Laxative group of glycosides: a- Sennoside A,B,C,D (Senna leaves and fruits). b- Cascaroside A,B (Cascara bark). c- Frangulin and glucofrangulin(Frangula bark). d- Aloin and barbaloin (Aloe vera and Aloe barbadensis juice). Cont… • Local irritant group: a-Sinigrin(Black mustered seeds_Brassica nigra) b-Sinalbin(White mustered seeds_Brasica alba) • Analgesics and antipyretics: Salicin hydrolysis Salisylic acid - Willow or Salix bark. • Keeping elasticity of blood vessels like: Rutin_Rutoside (Bitter orange peels, Lemon peels) • Anti-inflammatory group: a- Aloin for 1)acne 2)peptic ulcer b-Glycyrrhizin Classification of glycosides according to glycone part • Glucose _ glucoside group like in Sennoside. • Rhamnose _ Rhamnoside like in frangullin. • Digitoxose _ Digitoxoside like in digoxin. • Glucose and Rhammnose _ Glucorhamnoside _ glucofrangulin. • Rhamnose and glucose _ Rhamnoglucoside _ Rutin. Classification of glycosides on the basis of the linkage between glycone and aglycone part • O-glycosides : in these glycosides the sugar part is linked with alcoholic or phenolic hydroxyl or carboxyl group. • S-glycosides : in these glycosides the sugar attached to a Sulfur atom of aglycone such as in sinigrin. • N-glycosides : in these glycosides the sugar linked with Nitrogen atom of (-NH2,-NH-)amino group of aglycone like in nucleosides DNA,RNA • C-glycosides : in these glycosides the sugar linked (condensed) directly to Carbon atom of aglycone like in aloin. N.B C-glycosides are not hydrolyzed by acids or alkalis or by enzymes mainly . Classification of glycosides according to a glycone part : 1- if a glycone part alcohol -this group called alcoholic group like Salicin 2- if a glycone part aldehyde- this group called aldehydic gr. like glucovanillin. 3- if phenol called phenolic group like arbutin . 4-if cyanone called cyanogenic or cyanophoric or cyanoside like amygdalin. 5-if thio called glycosides or isothiocyanate glycoside like sinigrin or sinalbin (-S=C=N-) (SCN) 6-anthracene -------> anthraquinone glycoside –sennoside-. 7-steroid ------ steroidal glycoside (cardiac) Digoxin 8-flavone ,flavonol, flavanone –flavonoid glycoside 9-triterpenoid –saponin glycoside –glycyrrhizin ,melanthin (nigella seeds) or ginsenoside . Most of glycoside may be named according to the plant from which they isolated for example: 1-salicin –salix2-cascaroside _cascara 3-aloin- Aloe vera 4- sennoside – senna5-frangulin – frangula 6- glycyrrhizin – glycyrrhiza And others. Always glycosides founded in the plant with the enzymes which hydrolyzed them. We must damage these enzymes first to extract these glycoside by the following steps: 1-drying the plants fresh in special oven at 100 c for 30 minutes. 2-boiling them with organic solvents for 20 minutes 3- boiling them with acetone 5 minutes N.B. If present in this plant tannins or resins we add lead acetate to precipitate them. Classification of glycosides according to a glycone part. 1- phenolic group of glycosides:Such as arbutin which isolated from bearberry leaves Uses: UTI as antiseptic and antibacterial & mild diuretic Drugs : -Esoterica :cream :age spots -hydroquinone solution : wet hands 2- saponin group of glycoside a. Important group of glycosides which widely distributed specially in the higher plants parts b. Most of them are neutral compounds, soluble in water insoluble in the organic solvents. d. Irritant compounds for mucous membranes e. They form with hydrolysis glycone part which usually β-D-glucose or it’s acids (glucuronic acid) + Sapogenin f. Their aqueous solutions from froths (foam) on shakin and form emulsions on shaking and heating with oils and fats g. They destroy RBC (corpuscles) specially for fish and cold blooded animals m. Sapogenin (Triterpenoid) divided to : 1- steroidal Sapogenin 2- pent acyclic Sapogenin Examples:- licorice roots contain saponin glycoside which called Glycyrrhizin Glycyrrhizin hydrolysis glycyrrhetic acid + Z.M. glucyr acid Sweet taste : solution in H2O bitter taste insoluble in H2O expectorant anti-inflammatory Ginseng roots Panax roots Panax quinquefolius ,panax ginseng -contains saponin glycoside – ginsenoside ( panaxoside) Triterpenoid + steroidal nucleus Ginseng root uses : 1- stimulant 4-adaptogenic agent . Drugs : 1- geriatric pharmaton 2- gerimax 3- polyvit 2- Tonic 3- anti-stress Anthraquinone group of glycosides 1. They are anthracene derivatives (anthracene = is the main nucleus for anthraquinone compounds. 2. In the plant they biosynthesized from acetyl-CoA and malonyl-CoA. 3. They have cathartic property (laxatives and purgative but some of them have anti-inflammatory activity. 4. With hydrolysis they give aglycone part which is di,tri or tetra hydroxy anthracene derivatives. 5. They hydrolyzed only by acids or by enzymes, but not hydrolyzed by alkalines. 6.They have orange or red color (most of them) 7.Soluble in water, insoluble in the organic solvents. 8. They have bitter taste and slightly characteristic odor. 9. Anthraquinone may be free state compounds (free from sugar) or anthraquinone glycoside. Relationship between anthracene derivatives According to linkage between glycone part and aglycone part anthraquinone glycoside may classified to : 1.C-glycoside … very stable to hydrolysis. 2.O-glycoside … 3-o and c- glycoside. Alcoholic group of glycosides Such as: Salicin which obtained from Salix bark, Willow bark. Salicin hydrolyzed by: 1.Enzyme emolsin 2.Acid like HCl,HNO3 3.Alkaline solutions like NaOH The effect of salicylic acid : 1. 2. 3. 4. 5. 6. Analgesic. Anti-pyretic. Anti-coagulant (anticlotting agent). Anti- inflammatory activity (Rheumatism) Wart and corn remover Prevents colon cancer Aldehydic group of glycoside Such as : Glucovanillin which obtained from : Vanilla beans (fruits) ** Vanillin : volatile oil which used as flavoring agent ** vanillin : 1.phenolic group volatile oils 2.aldehyde group of volatile oils Isothiocyanate group of glycoside ( sulfur glycoside or thio glycoside ) 1. Group of glycosides which contains sulfur (Sglycoside) 2. Present in many cruciferous plants, on hydrolysis. 3. They produce isothiocynate (SCN) aglycone Such as : 1.sinigrin which founded in Black Mustard seeds 2.sinalbin which founded Brassica alba --White Mustard seeds. These plants contain also enzyme myrosin (myrosinase) which hydrolyze these glycosides. Properties of Mustard oil : 1.Irritant for mucous membrane 2.Volatile 3.Pungent 4.Characteristic odor The uses of Mustard seeds : counter irritant rubefacient, condiment, emetic in large doses. Drug :Acne aid soap®- Agis Treatment of Acne . Sinapine : alkaloids – alkaloidal amine group (protoalkaloid properties of Acrinyl isothiocynate. 1.Less irritant than allyl isothiocynate. 2.Less volatile. 3.Odorless. 4.Pungent. Uses of white mustard seeds Condiment, carminative, counter irritant, emetic Organic sulfur drugs Garlic cloves : Characteristic odor of Garlic Allicin : yellow liquid responsible for the odor of Garlic. Uses : 1.Anti-bacterial 2.Anti-hyperlipidemic activity HDL 3.anti-histaminic 4.anti-coagulant 5.Immune system stimulant. ** Coated tablet and capsules stomach acid inactivates alliinase enzyme. Cyanogenic glycosides Group glycosides which widely distributed between the members of Rosaceae family. They gave with hydrolysis HCN (Hydrocyanic acid) They derived from nitrile of mandelic acid. Cyanosides like : 1. Amygdalin : a. Bitter almond seeds. b. Apricots seeds. c. Plums seeds. d. Peaches seeds 2. Prunasin : present in Wild Cherry bark (( Prunus sertina)) Linmarin : Linseeds The plants contain enzyme emolsin which produce during hydrolysis two enzymes 1. Amygdalase hydro. Amygdalin. 2. Prunase hydro.Prunasin. Flavonoids Large group of glycosides which widely distributed in the plants kingdom and in all plants parts (leaves, roots, rhizomes, fruits peels) Various colors in flowers( yellow, orange, red, purple.) They are benzo-gama-pyrone derivatives. Their chemical structure based on (C6 C3 C6 ). The general uses of Flavonoids and Flavonoid glycosides. 1. Increase elasticity of blood vessels specially Rutin and hesperidin which known as vitamin (p) 2. Anti inflammatory activity like Taxifolin 3. 4. Anti spasmodic activity like Thyme and sage flavonoids. Cytostatic activity. Classification of Flavonoids according to the main nucleus Main nucleus flavonoids Flavonoids glycosides Flavonol unsaturated yellow quercetin Quercitrin Rhamnoglucoside Flavone (unsat) Yellow Diosmetin Vitexin Flavanone (satur) colorless Hesperitin Naringin ** C.G (Cardiac glycosides) : group of glycosides which has powerful action on cardiac muscles (cardiac muscle stimulant). C.G glycone part consist of one , two, three monosaccharide units or more similar or different Aglycone part has steroidal nucleus cyclopentanoperhudrophenanthrene B. glycone part of C.G always attached at C-3 position of aglycone part classification 1. cardenolide (one double bond, lactone ring) : Has five member lactone ring (unsaturated) attached at C17 B position of steroidal nucleus. 2. Bufadienolide: (contain two double bonds, lactone ring) Has six member ( unsaturated ) lactone ring attached at C-17 alpha – position What is important for activity of C.G :- 1) The presence of hydroxyl group in C14 position makes the glycoside very active and gives rapid action in the body, but if we change it to (H+) group the drug will be inactive or less active. 2) The presence of Alpha & Beta unsaturated lactone ring increases the activity of C.G, but if we make it saturated the C.G will lose its activity. 3) The ring junctions Cis, Trans, Cis make the nucleus very stable so more active. 4) The sugar part increases absorption and distribution of C.G in the body S.P : Glucose, Rhamnose, Cymarose, Digitoxose Examples of Cardenolides 1) Digitalis glycosides Digoxin Digitoxin Gitoxin 2) Strophanthus gratus C.G : Oubain 3) Strophanthus Kombe glycoside : K- strophanthin Bufadienolide example Squill bulb glycoside Scillaren ** Chemical tests :1) Keller Kiliani test : C.G + CH3COOH + H2SO4 + FeCl3 2) Legal test : C.G + pyridine sodium nitroprusside *Uses : 1-CHF brown Red to pink colour 2- arrhythmias * General properties of C.G : taste 1- Amorphous powder 2-bitter 3- sol. In H2O 4-Insol. In Org. solvents 5- Very toxic compounds 6- Odorless dig. Bind specific antibody Tannins • Complex natural organic compounds, polyphenolic, polyhydroxy benzoic acid derivatives or Flavanol derivatives. • Widely distributed in the nature (leaves, barks, immature fruits but, they disappear during ripening process). • They protect the plants from herbivorous animals, insects, bacteria and others specially during growth, like in new leaves which contain high percentage of tannins. • Some of them have low molecular weight but, most of them have very large molecular weight. Physico_chemical properties 1. They form colloidal solutions with water. 2. Non crystalline substances. 3. Their aqueous solution is acidic. 4. They have sharp puckering taste. 5. They have astringent property. 6. They precipitated by: alkaloids, gelatin, salts of heavy metals, proteins (enzymes). Tannins are classified into: 1.True tannins (hydrolysable)+ (nonhydrolysable). 2. Pseudo tannins. Classification of tannins: 1)True tannins: (a)Hydrolysable group: 1- Hydrolyzed by acids or enzymes (Tannase). 2- They are esters of a sugar (β-D-glucose) with one or more trihydroxybenzene carboxylic acid. 3-The tannins which derived from Gallic acid called Gallo-tannin or glucogallin like in Rhubarb rhizomes, Clove buds, Bearbers leaves. 4- tannis which derived from ellagic acid called ellagotannin or glucoellagin like in Pomegranate roots bark and Eucalyptus leaves (which also contain Tannic acid which is Gallotannin). 5- They form (H.T) with FeClз dark blue color. (b) Non hydrolysable group of tannin, condensed tannins or called proanthocyanidin tannins: 1- has resistance to hydrolysis by acids or enzymes. 2- they are Flavanol derivatives: 1) Flavan-3-ol like catechin 2) Flavan-4-ol like leucocyanidin 3- they are founded: In barks like: Cinnamon (Phlobatannins), Cinchona (Cinchotannin), Wild cherry. In seeds: Cacao, Cola (Colacatechin). In leaves: Tea. 4- they produce dark green color with FeClз. 5- they produce Catechol with HCl(conc.) and with vannillin_HCl reagent. 2) Pseudo tannins: 1- they have low molecular weight. 2- they occur: as Gallic acid like in Rhubarb rhizomes. as catechin like in Cacao, Acacia. as chlorogenic acid like in coffee. The general uses of tannins: 1)Anti diarrhea . 2) Anti bacterial . 3) Antidote in case of poisoning from alkaloids and heavy metals . 4) Haemostatic . 5) Mild diuretic . 6) Leather industry . 7) Astringent for inflammated mucous membrane 8) Stomachic . Chemical tests: 1) With FeClз : (a) H.T→ dark blue (b) Non H.T → dark green 2) ppt. with alkaloids, gelatin, proteins, and salts of heavy metals like lead acetate, copper acetate. 3) With H2SO4 → yellow 4) Vanillin_HCl reagent → red to pink color.