Solutions and Stoichiometry
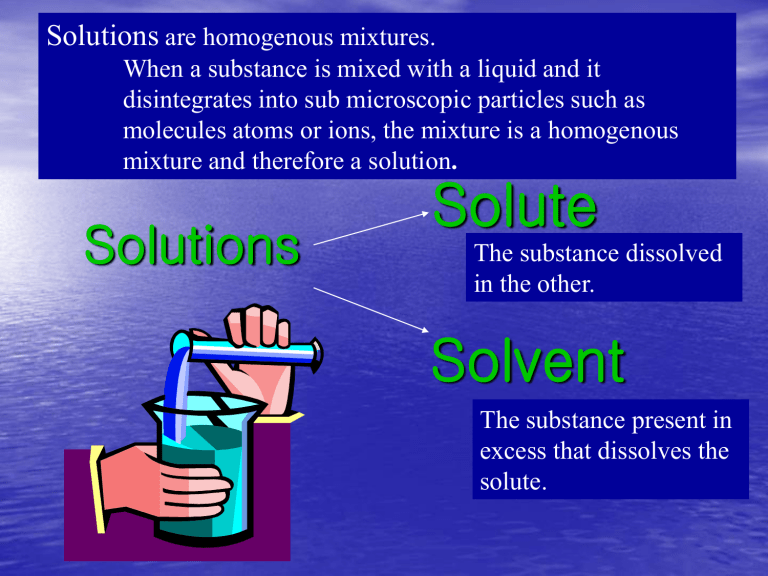
Solutions are homogenous mixtures.
When a substance is mixed with a liquid and it disintegrates into sub microscopic particles such as molecules atoms or ions, the mixture is a homogenous mixture and therefore a solution .
Solute
Solutions
The substance dissolved in the other.
Solvent
The substance present in excess that dissolves the solute.
Why is water called the universal solvent?
because it can dissolve most ionic and polar compounds.
Example of the dissolving process of an ionic compound in water
http://www.northland.cc.mn.us/biology/Biology1111/animations/dissolve.html
Vocabulary
Solubility : amount of substance that can be dissolved in a certain solvent.
Diluted: Contains small amount of solute for the given amount of solution.
Concentrated: Contains large amounts of solute for the given amount of solution.
Concentrated solution Diluted solution
Saturated solution: It cannot contain more solute at the given temperature.
Supersaturated solution: Contains more solute that the amount it can contain at the given temperature.
This is an unstable solution and the excess of solute will eventually separate, with any disturbance, or with the use of a seed crystal, from the solution forming a precipitate.
This process is called crystallization http://genchem.chem.wisc.edu/demonstrations/Gen_Chem_Pages/11solutionspag e/crystallization_from_super.htm
Concentration:
Is the amount of substance contained within a given volume of solution .
c
=
Amount of substance
Solution volume in dm 3
MOLARITY
Molarity is the concentration of a solution in moles of solute per liter of solution.
M
= n
V
L or
M
= n
V dm 3
• Other forms of concentration used are: % composition (parts per hundred) and ppm (parts per million)
• ppm is used when the concentration is very small (like the addition of fluorine to tap water)
% by mass
= g solute
X g solution
100 ppm = mg dm -3
• Sample Problems
Calculate the molarity of a solution prepared by dissolving
11.5g of solid NaOH in enough water to make 1.50 L of solution.
What do we need to find first?
The moles of NaOH…
11.5g NaOH
X
1
40.00
mol NaOH
= 0.288 mol NaOH g NaOH
Molar mass
Na: 22.99
O: 16.00
H: 1.01
40.00g/mol
M = n
=
V
(L)
0.288 mol NaOH
1.50L solution
= 0.192 mol L -1
• Sample Problems
What is the concentration in ppm of the solution in the previous problem?.
What do we need to find first?
The mg of NaOH…
11.5g NaOH
X
1,000
1 mg NaOH
= 11,500 mg NaOH g NaOH ppm = mg
V
(L)
=
11,500 NaOH
1.50L solution
= 7,670 ppm
Solve the problems in your notes
Dilution
When a solution is prepared dissolving a concentrated solution the dilution formula can be used.
Formula:
V c
M c
= V d
M d
Remember that you can only use this formula for dilution
Stoichiometry
Stoichiometry refers to the use of a balanced equation to calculate the mass of products and/or reactants.
Example:
How many moles and what mass of carbon dioxide and water can be obtained when 5.00g of ethane (C
2
H
6
) react with enough oxygen (Combustion reaction).
Steps
1. Write and balance the equation for the reaction
2. Convert the known (given) mass of the reactant or product to moles of the substance
3. Use the balanced equation to set the appropriate mole ratio
4. Use the ratio to calculate the number of moles of the desired reactant or product
5. Convert from moles back to grams if required by the problem.
Solve the problems in your notes
Limiting and excess reactants
Limiting reactant is the reactant that is totally consumed during the reaction and therefore controls the amount of products that can be formed.
Excess reactant is the reactant that doesn’t react completely during the reaction and therefore there is an excess of it remaining after the reaction.
Mg(s) + FeSO
4
(aq) ---- MgSO
4
2.00g
2.00g
(aq) + Fe(s)
Which one will react completely?
Example
Mg(s) + FeSO
4
(aq) ---- MgSO
4
(aq) + Fe(s)
2.00g Mg X 1 mol Mg
24.31g Mg
=
0.0823 mole Mg
0.0823 mole Mg X 1 mole FeSO
1 mol Mg
4 =
0.0823 mol FeSO
4
Moles of FeSO
4 needed to react completely with
0.0823 moles Mg
2.00g FeSO
4
X
1mole FeSO
4
151.92 g FeSO
4
=
0.0132 moles FeSO
Moles of FeSO
4
4 that we have
We need 0.0832 moles FeSO
4 to react with 2.00 g of Mg and we have only 0.0132 moles of FeSO
4
Which reactant is going to be totally consumed in the reaction?
FeSO
4 is the limiting reactant
It will be totally consumed during this reaction
It determines the amount of products that can be obtained
How much iron (Fe) will be obtained?
(explain)
Answer: 0.737g Fe
Mg is the reactant in excess. This means that at the end of the reaction there will be some magnesium left.
How much Mg will be left after the reaction?
(explain)
Answer: 0.320g Mg
Do prob.15 p 177. Students do 16
Molarity (review)
Molarity is the concentration of a solution in moles of solute per liter of solvent.
M
= n
V in dm
3
M= Molarity
N= number of moles of solute
V
L
= Volume in liters of solution.
Do prob.17and 18 p 179.
Percent YIELD
The real amounts of products formed during a chemical reaction are usually different from the expected amounts.
The real amount of one product obtained
(experimental) is called the ACTUAL YIELD
The expected amount, the amount of products calculated through the stoichiometry of the reaction, from the limiting reactant, is the THEORETICAL YIELD
Percent Yield =
Actual yield
Theoretical Yield
X 100 %
Do prob.19and 20 p 182.
combustion reaction to find the Empirical formula
A 0.496 g sample of an unknown hydrocarbon was completely burned in oxygen. The sample produced
1.56 g of carbon dioxide and 0.638 g of water.
a) How many moles of carbon dioxide were formed?
b) How many moles of water were formed?
c) What is the empirical formula of the hydrocarbon?
d) A 26.0 g sample of another unknown compound containing only carbon and hydrogen was burned in excess oxygen and 88g of CO
2 were produced. What is the possible molecular formula of this compound?