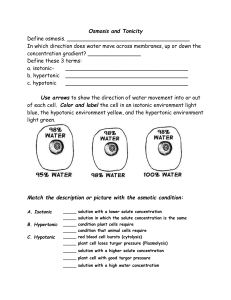
Lab 7. Diffusion - College Materials
advertisement

Chapter 9. Cellular Transport Mechanisms: Understanding Diffusion and Osmosis. Student Learning Outcomes At the completion of this exercise, the student will be able to: 1) 2) 3) 4) 5) 6) 7) 8) Compare and contrast passive and active transport mechanisms. Describe several means of passive and active transport. Provide specific examples of passive and active transport. Compare and contrast endocytosis and exocytosis. Define equilibrium Define solution, solvent, and solute. Compare and contrast hypotonic, hypertonic, and isotonic solutions. Describe cytolysis and plasmolysis. Overview: One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining the homeostatic state of the cell. If you recall, the plasma membrane is composed primarily of a phospholipid bilayer and specialized proteins. The unique structure of the plasma membrane allows it to be selectively permeable to certain substances. The permeability of the membrane is regulated by several variables, including size of the molecule, polarity of the molecule, and external conditions such as concentration, temperature, and pressure. For molecules to enter or exit a cell, they must overcome the concentration gradient, which involves the movement of molecules from regions of greater concentration to regions of lesser concentration. Living systems have two primary mechanisms for moving substances in and out of the cell – passive and active transport. In passive transport the cell uses no energy (ATP) as essential substances are moved across the plasma membrane. Examples of molecules moved by the various means of passive transport are oxygen, water, and glucose. The most fundamental means of passive transport is diffusion, the random movement of molecules from regions of greater concentration to regions of lesser concentration. This random movement also is known as Brownian motion. A state of equilibrium is attained when an equal distribution of molecules exists throughout the system. The movement of water across the plasma membrane in living systems is called osmosis. Other mechanisms of passive transport include facilitated diffusion and filtration. 1) In facilitated diffusion, carrier proteins along the cell membrane are required to ferry specific molecules such as glucose across the membrane into the cell. 2) Filtration involves hydrostatic pressure (water pressure) forcing molecules through a cell membrane. Filtration is an essential mechanism that takes place in the kidneys in the formation of urine. 1 In active transport, energy in the form of ATP is required for the movement of substances across the concentration gradient. Biological pumps essential in this process are the following: 1) The proton or hydrogen pump is necessary to maintain the normal pH of the stomach. 2) The calcium pump is important in nerve and muscle function, and 3) The sodium – potassium pump is integral in cellular metabolism. Macromolecules such as polypeptides and polysaccharides are too large to traverse the cell membrane by either passive of pump systems. Instead, they must be transported into the cell by endocytosis and out of the cell by exocytosis. Both endocytosis and exocytosis employ the formation of vesicles in transporting substances. In endocytosis, solid substances can be engulfed by a cell through phagocytosis. Everyday examples in biological systems include the ingestion of food particles by an amoeba and the engulfing of foreign particles by a macrophage. Another example of endocytosis is pinocytosis, or cell drinking, in which liquid droplets that may contain salts and other molecules are taken into the cell through vesicle formation. Specialized cells in the roots of plants use pinocytosis to ingest a variety of nutrients. 2 TAKE A WHIFF The instructor will spray perfume or room deodorizer in a front corner of the classroom. The molecules will diffuse throughout the room in an attempt to attain a state of equilibrium. Timing devices are used to determine the rate of diffusion of the molecules across the room. PROCEDURE 9.1 SMELL 1) Students should be equally dispersed throughout the classroom. 2) The instructor sprays a small amount of a scent in the front corner of the room and this will serve as time zero. 3) Students will record the time and raise their hands when they detect the odor. 4) Students discuss their results. Q1. How long did it take for the scent to reach the farthest point? _____________________________________________________________________________________ Q2. What variables can affect the rate of diffusion of the scent? _____________________________________________________________________________________ WHAT IS IN THE BAG? In this activity, dialysis tubing will serve as the selectively permeable membrane. After making a bag with the dialysis tubing and filling it with colorless cornstarch solution, the bag will be immersed in a beaker containing an iodine solution that is caramel in color. Movement of the iodine molecules across the membrane can be detected by a change in the cornstarch solution to a purplish – brown color. Materials 250 mL beaker 25 mL graduated cylinder Cornstarch solution Iodine solution Water Glass rod Dialysis tubing String Timing device Ruler Paper towels PROCEDURE 9.2 MEMBRANE 1) 2) 3) 4) 5) 6) 7) Obtain glassware and needed accessory materials. Measure and cut a 15 cm length of dialysis tubing. Place the tubing in water until it becomes soft and pliable. Using string, form a bag with the dialysis tubing closing one end. Fill the dialysis tubing bag halfway with the cornstarch solution. Using string, tie off the top of the bag. Immerse the bad containing the cornstarch solution into a beaker containing 200 mL of iodine solution and record the time and the color of the solution. 3 Figure 1 Method for filling dialysis tubing. 1. What color is the iodine solution? __________________________________________________________________________ 2. What is the color of the cornstarch solution? __________________________________________________________________________ 8) Leave the experiment undisturbed for 15 minutes. 9) After the elapsed time, remove the bag from the solution and place it on a paper towel. 10) Observe the color changes in the dialysis bag. 3. What color changes did you observe in the bad and in the solution. __________________________________________________________________________ 4. Explain the reason for such color changes in both the bag and solution. In the space provided below, graphically illustrate the results of the activity. 4 OSMOSIS ACTIVITIES Recall that osmosis is the diffusion of water across a selectively permeable membrane, and diffusion always occurs from regions of greater concentration to regions of lesser concentrations. A typical solution consists of two components – the solvent as the dissolving medium and the solute as the substance dissolved in the solvent. In a saltwater solution, the water serves as a solvent and the salt as the solute. Tonicity refers to the concentration of solute in the solvent. 1) In a hypotonic solution, there is a lower concentration of solute relative to the inside of the cell. If a cell such as a red blood cell or a potato cell is placed in a hypotonic solution, water will rush into the cell in attempt to reach a state of equilibrium. An ideal hypotonic solution is distilled water, because it devoid of solutes. As the cell begins to fill with solvent, the cell will swell, and perhaps burst. The bursting of cells in a hypotonic solution is called cytolysis. 2) In a hypertonic solution, there is a higher concentration of solute relative to the inside of the cell. If a cell such as a red blood cell or a potato cell is placed in a hypertonic solution, water will be drawn out of the cell into the outside solution in an attempt to teach a state of equilibrium. This is called crenation in red blood cells and plasmolysis in plant cells. In plants, the swelling of cells placed in a hypotonic solution results in turgor pressure. The framework cell wall protects the cell from bursting. Turgor pressure keeps the plant erect. If the turgor pressure is lost in a plant, the plant will wilt. Just think of the plants in your yard on a hot summer’s day. Many cells exist in an isotonic solution, which has the same concentration of solute outside and inside of the cell. Normally, red blood cells exist in an isotonic state in plasma. Many organisms, such as some species of sharks, can undergo osmoregulation, in which they can alter their internal solutions to equal that of the environment to maintain homeostasis. This explains why some species of sharks can be found living in freshwater rivers near coasts. 5 PLANT TISSUE: HYPOTONIC VS. HYPERTONIC Testing Tissues in Hypotonic and Hypertonic Solutions Plant tissues In this activity, strips of potato tissue will be placed in hypotonic and hypertonic solutions, and observations will be made and recorded. Materials Potato strips 3 test tubes Test tube rack Sharpie Water Distilled water 10% sodium chloride solution Paper towels Forceps Scalpel Microscope slides and cover slips Microscope PROCEDURE 9.3 PLANT TISSUE 1) 2) 3) 4) 5) 6) Obtain the needed equipment and bring it to your lab station. With a marker, label the tubes “Control”, “Hypotonic Solution”, and “Hypertonic Solution”. Cut three strips of potato, each about 4 cm in length and 1 cm wide. Place each strip in a labeled test tube. Add tap water to the test tube labeled “Hypotonic Solution”, totally immersing the potato strips. Add 10% sodium chloride solution to the test tube labeled “Hypertonic Solution”, totally immersing the potato strip. 7) Add distilled water to the test tube labeled “Hypotonic Solution”, totally immersing the potato strip. 8) Let the solutions sit quietly for 30 minutes before making your observations. 9) Remove the potato strips and place them on a paper towel. Record your observations. 10) Using the scalpel carefully cut a thin piece of tissue from each strip. 11) Prepare a wet mount of each strip. 12) Using the microscope, observe each slide at 400X and record your results. 13) Clean your lab station 6 Sketch and Label your microscopic observations: 1) Potato strip in control (tap water) solution. 2) Potato strip in Hypotonic (ddH2O) solution 3) Potato strip in Hypertonic (10% NaCl) solution 7 1. Which strip was most limp? Why? ___________________________________________________________________________ ___________________________________________________________________________ 2. Which strip was most stiff? Why? ___________________________________________________________________________ ___________________________________________________________________________ CHICKEN EGG: HYPOTONIC vs. HYPERTONIC This activity is designed for you to do in your home or dormitory. Before engaging in this activity, review the definitions of hypertonic and hypotonic solutions. Acquire the materials and follow the procedure below. Materials Raw egg White vinegar Green food coloring Light corn syrup 2 – quart Mason jars or similar containers PROCEDURE 9.5 VINEGAR 1) 2) 3) 4) 5) 6) 7) Obtain the needed materials. Fill a Mason jar or container approximately halfway with white vinegar. Add 3 – 5 drops of green food coloring to the vinegar. Carefully place the entire egg into the vinegar solution. Set the experiment aside at least 8 hours Observe the egg Using a digital camera, video camera, or phone camera, or sketch (draw) to record the egg 1. Describe the egg ______________________________________________________________________________ ______________________________________________________________________________ 2. Using your knowledge of solutions, describe what has happened to the egg. ______________________________________________________________________________ ______________________________________________________________________________ 8 PROCEDURE 9.6 CORN SYRUP 1) 2) 3) 4) 5) 6) Obtain the needed materials Fill a Mason jar or container approximately halfway with light corn syrup. Carefully place the entire egg into the corn syrup. Set the experiment aside for at least 8 hours Observe the egg Using a digital camera, video camera, or phone camera, record the egg. 1. Describe the egg ______________________________________________________________________________ ______________________________________________________________________________ 2. Using your knowledge of solutions, describe what has happened to the egg. ______________________________________________________________________________ ______________________________________________________________________________ Why do Sea Turtles Cry??? Have you ever watched a nature show and seen sea turtles crying as they lay their eggs on some lonesome beach? Why do turtles cry? Is it because they are anticipating the future of their young, or perhaps they are sentimental, or maybe it hurts? No, it is not any of these reasons. Marine turtles have glands in the regions of their eyes that help them remove excess salt consumed from the hypertonic solution in which they live. That is why these turtles appear to cry! 9 Selective Permeability of Membranes Osmosis occurs when different concentrations of water are separated by a selectively permeable membrane. One example of a selectively permeable membrane within a living cell is the plasma membrane. Dialysis tubing is a selectively permeable material that provides a means to demonstrate the movement of substance (ions or large molecules?) through cellular membranes. The dialysis tubing is a selectively permeable cellulose sheet that permits the passage of water and ion molecules but obstructs the passage of larger molecules such as starch and proteins. If you examined this membrane (dialysis tubing) with a scanning electron microscope, you would see that is POROUS; it thus prevents molecules larger than the pores from passing (diffusing) through the membrane. Materials Per Group: One 15 cm length of dialysis tubing, soaking in ddH2O Two 10 cm pieces of string Dishpan half-filled with ddH2O 400 mL beaker Ring stand and funnel apparatus 8 test tube Test tube rack Sharpie 25 mL graduated cylinder Lugol’s (I2KI) solution 2% barium chloride (BaCl2) solution 2% silver nitrate (AgNO3) solution Biuret reagent Scissors Per Lab: Bottle of 1% soluble starch in 1% sodium sulfate Bottle of 1% albumin in 1% sodium chloride 4 test tubes demonstrating positive test for starch, sulfate ion (SO4-2), chloride ion (Cl-), and protein (Egg albumin). Procedure 1) Obtain a 15 cm section of dialysis tubing that has been soaking in ddH2O. 2) Tie one end of the bag with a string or dental floss to form a leak proof bag. 3) Slip the open end of the bag over the stem of a funnel and fill the bag approximately half full with 15 ml of a solution of 1% soluble starch in 1% sodium sulfate (Na2SO4). 4) Remove the bag from the funnel and tie the open end of the bag. (Make sure there is no air in the bag) 5) Rinse the tied bag in a dishpan filled with ddH2O. 6) Pour 200 mL of a solution of 1% albumin in 1% sodium chloride (NaCl) into a 400 mL beaker. 7) Place the bag into the beaker. 8) Record the time. ___________________ 9) With a sharpie, label eight test tubes, numbers 1-8 10) 75 minutes after the start of the experiment, pipet 3 mL of the solution that is in the beaker to test tube 1-4. 11) Perform the following tests. Your instructor will provide the positive controls. Record your result on table 9.1. a. b. c. d. Test for starch. Add 2-3 drops of Lugol’s solution (I2KI) to test tube 1. Test for sulfate ions. Add 10 drops of 2% barium chloride solution (BaCl2) to test tube 2. Test for chloride ions. Add 10 drops of 2% silver nitrate solution (AgNO3) to test tube 3. Test for protein. Add 10 drops of Biuret reagent to test tube 4. 10 12) Remove the bag from the beaker and rinse the bag in the dishpan with ddH2O. 13) Using scissors cut open and empty the solution in a graduate cylinder. 14) Pipet 3 mL of the solution that is in the gradate cylinder to test tube 5-8. 15) Perform the following tests. Your instructor will provide the positive controls. Record your result on table 9.2. a. b. c. d. Test for starch. Add 2-3 drops of Lugol’s solution (I 2KI) to test tube 5. Test for sulfate ions. Add 10 drops of 2% barium chloride solution (BaCl2) to test tube 6. Test for chloride ions. Add 10 drops of 2% silver nitrate solution (AgNO3) to test tube 7. Test for protein. Add 10 drops of Biuret reagent to test tube 8. 16) Discard content of test tubes, graduated cylinder, and beaker down the sink drain. Wash glassware and return them to your station. Table 9.1 Result of tests for substances in the beaker Substances At start of experiment After 75 min. Starch Sulfate ions Chloride ions + Protein (egg albumin) + Content of beaker: (+) = Presence. (-) = Absence Table 9.2 Result of tests for substances in the Dialysis bag Substances At start of experiment After 75 min. Starch + Sulfate ions + Chloride ions Protein (egg albumin) Content of beaker: (+) = Presence. (-) = Absence 1. To which substances was the dialysis tubing permeable? _________________________________________________________________________ _________________________________________________________________________ 2. What physical property of the dialysis tubing might explain its differential permeability? _________________________________________________________________________ 3. To which substances (molecules) was the dialysis tubing impermeable? ________________________________________________________________________ 11 Plasmolysis in Plant Cells Plant cells are surrounded by a rigid cell wall composed primarily of the glucose polymer, cellulose. Many plant cells have a large central vacuole surrounded by the vacuolar membrane. The vacuolar membrane is selectively permeable. Normally, the solute concentration within the cell’s central vacuole is greater than that of the external environment. Consequently, water moves into cell, creating turgor pressure, which presses the cytoplasm against the cell wall. Such cells are said to be turgid. Many non-woody plants (like beans and peas) rely on turgor pressure to maintain their rigidity and erect stance. In this experiment, you will discover the effect of external solute concentration on the structure of plant cells. Materials Compound Microscope Forceps Microscope slides and coverslips Elodea leaf Bottle of ddH2O Bottle of 20% Sodium Chloride (NaCl) Paper Towel Procedures: 1) With a forceps, obtain two healthy elodea leaves. 2) Place one piece of leaf on a microscope slide and add one drop of ddH2O and place a coverslip on top of the leaf. Place a paper towel on top of the slide and tap around the coverslip to extract excess water (See “wicking” technique below). The technique of "wicking" is used to draw a solution across the specimen on a slide. By placing a piece of tissue or paper towel at one edge (right) and dropping the solution at the edge of the other side (left), the solution is drawn or "wicked" across the specimen. 12 3) View the slide of the elodea leaf in ddH2O through a compound microscope. Focus the slide with the low-power objective. Once focus add a drop of oil immersion and switch the objective to oil immersion (1000X). Label the pointing structures below. Figure 1 above shows normal Elodea. The chloroplasts are spread throughout the cell before the salt solution, after the distilled water is put onto the slide. Distilled water represents a hypotonic solution, yet the cells do not burst because of the cell wall. 4) Once observed switch to low-power objective and remove the slide. 5) After completion of the activity, clean up your work area and return or dispose of the materials as instructed. 13 6) Place the other piece of leaf on a microscope slide and add one drop of 20% Sodium Chloride (NaCl) and place a coverslip on top of the leaf. Place a paper towel on top of the slide and tap around the coverslip to extract excess water. Then, view the slide of the elodea leaf through a compound microscope. Focus the slide with the low-power objective. Once focus add a drop of oil immersion and switch the objective to oil immersion (1000X). After a few minutes, the cell will lose water this process is called plasmolysis (See figure 2 below). Label the pointing structures below. 7) Once observed switch to low-power objective and remove the slide. 8) After completion of the activity, clean up your work area and return or dispose of the materials as instructed. 14 Tonicity illustrates one solution’s solute concentration evaluates to that of another solution. The solution containing the lower concentration of solute molecules than another is hypotonic relative to the second solution. Solutions containing equal concentrations of solute are isotonic to each other, while one containing a greater concentration of solute relative to a second one is hypertonic (See picture below for tonicity in a plant cell). 1. Was the inside of the central vacuole in the elodea leaf hypotonic, isotonic, or hypertonic compared to the drop of ddH2O that was added (outside of the cell or environment)? ______________________________________________________________________________ 2. Was the 20% NaCl solution added (outside of the cell or environment) hypertonic, isotonic, or hypotonic relative to the cytoplasm? ______________________________________________________________________________ 3. In what direction will the water move if a hypotonic and a hypertonic solution are divided by a selectively permeable membrane. ___________________________________________________ 4. Name three selectively permeable membranes that are present within the elodea cells and that were involved in the plasmolysis process (see figure 3 on page 14)? a) _____________________________________________________________________ b) _____________________________________________________________________ c) _____________________________________________________________________ 15 ___________________________________________ Last Name, First Name [lab partner N0. 1] ____________________________________________ Last Name, First Name [lab partner N0. 2] _______________________________ _______________________________ Last Name, First Name [lab partner N0. 3] ___________________________ Section Last Name, First Name [lab _______________ partner N0. 4] ____________________ group # Date Review Questions Chapter 9: Cellular Transport Mechanisms: Diffusion and Osmosis 1. Why does the cell membrane have to be selectively permeable? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. Compare and contrast active and passive transport? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 3. What variables affect the rate of diffusion in biological systems? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 16 4. Describe osmosis in your own words. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 5. What is the difference between pinocytosis and phagocytosis? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 6. Explain why a sailor set adrift cannot drink seawater? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 7. If a medical technologist notes that many red blood cells are crenate, what are possible sources that could have caused this phenomenon to occur? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 17 8. List and describe three active transport pumps that function in biological systems. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 9. Draw and then describe what happens to a red blood cell when placed in a hypotonic solution, a hypertonic solution and an isotonic solution. 18 LEADING TO RESEARCH 1. Discuss specific adaptations of marine fishes that allow them to survive in saline environments. Why ate marine organisms said to literally live in a desert? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. Discuss specific adaptations of marine fishes that allow them to survive in saline environments. Why are marine organisms said to literally live in a desert? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 3. What is the difference between an osmoregulator and an osmoconformer? Give examples of each. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 19