CHAPTER 6: Chemical Names and Formulas
advertisement

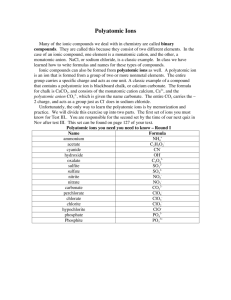
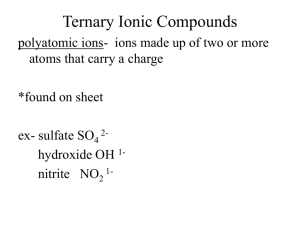
Ionic Compounds A Review Ion: atom or group of atoms that have a positive or negative charge Ions form when an atom or group of atoms gains or loses electrons METALS lose electrons to form positive CATIONS. NONMETALS gain electrons to form negative ANIONS Having One Atom! Monatomic Ions Ions consisting of only 1 atom – The charge for ions formed from representative elements can be determined by looking at the periodic table Group 1A metals form cations with a 1+ charge Group 2A metals form cations with a 2+ charge Group 3A metals form cations with a 3+ charge Group 5A nonmetals form anions with a 3- charge Group 6A nonmetals form anions with a 2- charge Group 7A nonmetals form anions with a 1- charge Group 8A elements do not form ions Having Many Atoms! Polyatomic Ions Tightly bound groups of atoms that behave as a unit and carry a charge Examples of most popular: 1- charge: NO3- NO2- OH- ClO3- C2H3O2CO32SO42SO32 2- charge: 3- charge: PO43+ NH 1+ charge: 4 Naming Monatomic Cations The name of a cation is the SAME as the name of the element!! Ex: Na atom forms a sodium cation Ex: Li atom forms a lithium cation How would you name the following ion: Al+3 Aluminum ion Naming Monatomic Anions The name of a MONAtomic ion ends in “ide” Ex: Chlorine atom gains 1 electron to become the CHLORIDE anion Ex: Oxygen gains 2 electrons to become an anion named OXIDE What would we name the following ion? S-2 Sulfide ion Practice: What do we call: – A Barium atom when it loses 2 electrons Barium ion – An iodine atom when it gains 1 electron Iodide ion – A phosphorus atom when it gains 3 electrons? Phosphide ion Naming Ionic Compounds The cation comes 1st and keeps its name. Monatomic anions end in “ide” Polyatomic cations and anions keep their names. K2SO4 LiCl Lithium Chloride Potassium Sulfate Magnesium Fluoride MgF2 LiNO3 Lithium Nitrate MgCO3 Be(OH)2 Beryllium Hydroxide NH4Cl Ammonium Chloride Magnesium Carbonate Writing the Formulas! Cations and anions combine in a whole number ratio that produces a neutral compound. The total positive charge of the cations will equal the total negative charge of the anions. For example: 1 Al+3 combines with 3 F-1 (1 x +3) cancels with (3 x -1) Criss Cross Method 3 Step Process for Writing Ionic Formulas 1. Write the ions with their charges The charges are known from the periodic table or memorized for polyatomic ions. 2. Cross the charge numbers (dropping the + or -) to form the subscripts. (Subscripts of 1 are not written.) 3. Simplify the subscripts to the lowest whole number ratio Writing Formulas for Ionic Compounds Write the formula for magnesium chloride. 1. Write the ions with their charges The charges are known from the periodic table. Magnesium is in Group 2A and forms a 2+ charge. Mg2+ Mg Cl1- Cl Chlorine is in Group 7A and forms a 1- charge. 2. Cross the charge numbers (dropping the + or -) to form the subscripts. (Subscripts of 1 are not written.) MgCl2 3. Simplify the subscripts to the lowest whole number ratio Writing Formulas for Ionic Compounds Write the formula for beryllium oxide. 1. Write the ions with their charges The charges are known from the periodic table. Beryllium is in Group 2A and forms a 2+ charge. Be2+ Be O2- O Oxygen is in Group 6A and forms a 2charge. 2. Cross the charge numbers (dropping the + or -) to form the subscripts Be2O2 3. Simplify the subscripts to the lowest whole number ratio. (If both subscripts are the same, they can be dropped.) BeO Writing Formulas for Ionic Compounds With Polyatomic Ions Follow the same 3 steps. Just add parentheses around the polyatomic ion before it gets a subscript Writing Formulas for Polyatomic Ionic Compounds Write the formula for calcium hydroxide. 1. Write the ions with their charges Calcium is in Group Ca2+ 2A and forms a 2+ Ca charge. OH1OH Hydroxide is a polyatomic ion that has a 1- charge. 2. Cross the charge numbers (dropping the + or -) to form the subscripts 3. Subscripts of 1 are not written 4. Simplify the subscripts CaOH2 There are 2 Hydroxide ions not just 2 Hydrogen atoms. Ca(OH)2 5. Remember to add parentheses around the polyatomic ion if a subscript has been added! Try Some Na2CO3 Sodium Carbonate Potassium Hydroxide KOH Magnesium Phosphate Mg3(PO4)2