Unit 10 – Solids and Liquids
advertisement

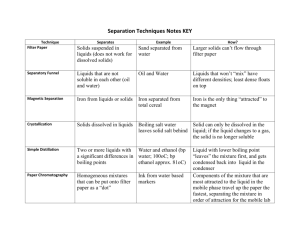
Unit 10 - Gases, Liquids and Solids General Properties: I. Gases: 1. Expansion 2. Compressible 3. Fluid 4. Low density 5. Diffusion 6. Effusion 7. Condense to liquid 8. No definite shape 9. No definite volume 10. Change volume with Temperature 11. Change volume with Pressure 12. Deposition (to solid) Fluid: Substance that can flow and take shape of container II. Liquids: 1. Definite volume 2. Fluid 3. High density 4. Diffuse 5. Incompressible 6. Dissolve solids 7. Surface tension 8. Boil / evaporate 9. Solidify III. Solids: 1. Definite shape 2. Definite volume 3. Not fluid 4. Melt 5. High density 6. Incompressible 7. Slow diffusion 8. Sublimation (solid to gas) 9. Rigid form Diffusion: move from area of high concentration to low concentration Kinetic Properties (KMT): (Movement) I. Gases: 1. Tiny particles 2. Constant straight line motion 3. Elastic collisions 4. Little or no attraction forces 5. Average kinetic energy KE = ½ mv2 II. Liquids: 1. Tiny particles 2. Constant motion (limited) 3. Elastic collisions 4. Some intermolecular attractions 5. Closely fit together III. Solids: 1. Tiny particles 2. Constant vibratory motion 3. Strong intermolecular forces 4. Rarely move position 5. Closely packed (fixed position) Examples of Gases, Liquids, and Solids Gases: elements and compounds Elements: a) monatomic gases – He, Ne, Ar, Kr, Xe, Rn b) diatomic gases – H2, N2, O2, F2, Cl2 Compounds: CO, CO2, NO, NO2, N2O, N2O3, NH3, C2H6, C3H8, SO2, SO3, AsH3 ….. Liquids: elements and compounds Elements: Hg, Br Compounds: HOH, C3H2OH, C3H5(OH)3, C2H5OH, C8H18 … Solids: elements and compounds Elements: most metals (Except Hg) nonmetals P, S, I, C Compounds: NaCl, NaHCO3, CuSO4, MgSO4, AlNa(SO4)2, C6H12O6, C12H22O11… Types of Solids 1) Crystalline: crystal lattice (3-D) Shapes: unit cells – cubic, body center or face center Basic crystal systems: a) isomeric cubic d) orthorhombic b) tetragonal e) monoclinic c) trigonal f) triclinic g) hexagonal Types of Crystals 1) Ionic – Hard, Brittle, High melting pt. examples: NaCl, CuSO4, AgNO3 2) Covalent – Soft, Low melting pt. examples: NH3, HOH, CH4 3) Network - hardness vary, High MP examples: diamond, graphite, quartz 4) Metallic – MP range, hardness range examples: Cu, Fe, Al,… Forming Crystals: evaporation or from magma SLOW cooling: large perfect crystal Defect: flaws (mistake in crystals) a) foreign atom /ion (changes color ) b) internal misalignment (fuzzy) c) dislocation - edge - screwed Edge dislocation: extra layer of atoms extends part of the way into a crystal Screwed dislocation: unequal growth while the crystal form 2) Microcrystalline Fullerines / Buckyballs contain carbon (graphite)16–128atoms sulfur 4 – 8 atoms phosphorus 30 +/- atoms Properties: strong, durable, hollow, fluffy shapes are spheres or tubes network bonding Examples: tennis racket frames golf club shafts airplanes frame / outer covering Types of Solids: 3) Amorphous: is also called meta-stable liquids or super -cooled liquids Properties: -melting pt range -weak intermolecular forces -temperature sensitive -random molecular arrangement Examples: Glass, Rubber, Plastics, Waxes Phases Below o 0C Phase Changes I. Chart- G Evaporate/ Condense boil Deposition L Sublimation Solidify Melt S II. GraphT release energy E M solidify melt P condense boil L G add energy S ENERGY III. Diagram4 P S 1. Melting Pt 2. Boiling Pt L 3. Triple pt 1 2 1 atm G 3 Temp 4. Critical pt Terms Melting: solid to liquid (add heat) Evaporation: liquid to gas without boiling Boiling: change of liquid to bubbles of vapor that appear throughout the liquid Condensation: gas to liquid (release heat) Solidification/Freezing: liquids to solids Sublimation: solid to gas without becoming a liquid Ex: I2, CO2, paradichlorobenzene Deposition: gas to solid without passing liq. Get your thinking caps on this will be FUN!!!!!! Holy Moley!!!!! Le Chatelier & Stress I’ll start will an easy concept! Equilibrium (Le Chatelier & Stress) Open System: Evaporation Room Temp Condensation cool Closed System: Dynamic Equilibrium: evaporation = condensation at one specific temperature Equilibrium: Two Opposing changes occur at equal rate Boiling Point Boil at same temperature until all liquid has vaporized Vapor pressure=atmospheric pressure Boiling Point changes with Pressure and / or Altitude changes : Increase pressure, BP (pressure cooker) Decrease pressure, BP (high Mt range) BOILING and ELEVATION • • • • • • • DEATH VALLEY CA HAZLET NJ BOULDER CO LEADVILLE CO MT WHITNEY CA MT McKINLEY CA MT EVEREST TIBET 100.3C 100.0C 94.0C 89.0C 85.0C 79.0C 70.0C Stress Heat or Cool: HEAT Explosion COOL Implode Equilibrium will shift to ease stress Water Ocean (saltwater), river, lakes and glaciers (freshwater), cover about 75% of earth’s surface. Living things are 70% - 90% HOH. Physical Properties of Water: 1. Ice(s), Water(l), Vapor(g) 2. Angular molecule O 1050 H H 3.Colorless, transparent, odorless, tasteless 4. Intermolecular forces (Hydrogen bond) 5. Highly polar 6. Rigid structure as solid “hex” shape 7. Most dense 4oC 8. FP 0oC / BP 100oC at STP 9. D(l) = 1.00 g/cm3 10. D(s) = .917 g/cm3 Ice floats in water 11. D(g) = .000748 g/cm3 as vapor 12. Hf = 334 joules/g; Hv = 2260 j/g 13. Csp = 4.18 j/goC (l); 2.06 j/goC (s) ; 2.02 j/goC (g) 14. Universal solvent Chemical Properties of Water: 1. Stable under standard conditions (STP) STP= standard temperature (0oC) and pressure (1 atm) 2. React with active metals H2 2 Na + 2 HOH 2NaOH + H2 3. It decomposes to H2 and O2 4. Metal oxide + HOH Bases BaO + HOH Ba(OH)2 5. Nonmetal Oxide + HOH Acids SO3 + HOH H2SO4 6. It promotes chemical changes. Aqueous reactions Used as a Standard for: 1. Temperature at sea level(thermometer) 2. Pressure (Barometer) 3. Volume (Liter) 4. Mass (Gram) 5. Density (specific gravity) 6. Heat (calorie/joule) Heavy Water: D2O (deuterium oxide) a) 2400 liters HOH 83 ml D2O b) more dense d= 1.2 g/cm3 c) BP 101.4 oC / MP 3.8 oC d) used as “tracer” in chem RXNs chemical and biological Terms: 1. Water of crystallization: homogeneous particles bounded by surface making definite angles. The slower the crystals form, the more perfect they are. 2. Hydrated crystal: a crystallized substance containing HOH 3. Anhydrate: substance without water 4. Effervescence: rapid evolution of small gas bubbles 5. Efflorescence: hydrated crystals lose HOH when expose to the air Ex: Na2CO3.10HOH fast process CuSO4.5HOH slow process 6. Deliquence: take up water from the air Ex: NaOH fast / CaCl2 slow 7. Hydroscope: insoluble material take up water vapor from the air Ex: hair, wool, silk 8. Miscible: two liquids can dissolve freely in one another in any portion. Ex: water + isopropanol 9. Immiscible: two liquids are not soluble in each other. Ex: water + oil 10. Effuse: gas particles pass through a tiny opening 11. Viscosity: the resistance of a liquid to flow. Ex: syrup 12. Lattice: 3-D arrangement of particles of a crystal 13. Unit cell: 3-D pattern of the entire lattice (repeating pattern) Holy Moley Math!!!!!!!!!!!!!!!! MATH CONCEPTS Remember the rules sig fig sig fig sig fig sci not sci not sci not UNITS Csp = specific heat capacity; energy needed to raise 1.00 g of substance 1.0 oC metals – low Csp nonmetals – moderate Csp compounds – varied Csp H = m x Csp x T H: energy in calories or joules Csp: heat capacity m: mass T: change in temp. Ex: A 15.00 g sample of HOH is raised from 21oC to 37 oC. How much energy is needed? H = 15.0 g x 4.18 j/goC x (37-21) oC = 1003 joules FIN This is what you need for Heavy Water