ppt file for this lecture (click to file)
advertisement

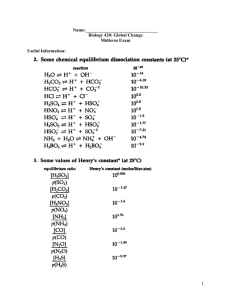
Lecture 6 Geosc 040 Chemistry of SeaWater –Dissolved Gas & Density Atmospheric Circulation & Ocean Circulation Today’s music: Duke Dumont Ocean Drive Radioactive Swimming Pools - Isosine Thanks to Andrew G, Hannah K, & Pamela 10,000 Emerald PoolsBORNS Home By the Sea Genesis Read the Course Materials We have spies everywhere! • Lecture Review Materials and Reading: • Homework 1: Problem w/ data server! If you completed it before Tuesday afternoon, you’re all set. If not, use the new data server. Same questions. New Due date is Sat. 30th of Jan by 5pm • Sign up to take Quiz 1 on Monday (Feb 1) • Cell Phone Recycling The Sea Around Us The Sea Around Us Ocean Surface Currents Density of Seawater The density of seawater is determined by temperature and salinity Density of Seawater Recall that Oceans have about 35 grams salt per kg water (salinity = 35 ppt or 35 PSU) (PSU= Practical Salinity Units) • Density of seawater increases as •Temperature drops •Salinity increases • Maximum density and freezing point coincide for 24.7 ppt •Note that ppt = %O • At higher salinities seawater reaches maximum density at freezing point Density of Seawater & Stratification Surface Zone (mixed layer) Zone of rapid change with increasing depth Pycnocline is the zone of rapid density change with depth • Temperature and salinity vary considerably in the ocean • Because water has low viscosity and can flow, denser water masses sink while less dense water masses rise to the surface • Deep water masses have a rather narrow range of T and S • Most T and S variability occurs in surface or near-surface waters • The ocean is density stratified (this is a stable configuration) What is the residence time of deep water in the large ocean basins? 500 to 2000 years! t= Residence time of water in the ocean Volume = 1.4 x 109 km3 River Influx = 3.7 x 105 km3 /yr t = Volume / Influx How long does it take to cycle ocean water through rivers and back again? 1.4 x 109 km3 t= 3.7 x 105 km3/year t ≈ 4000 years Today’s in-class iClicker exercises: A) Full credit if you answer 75% or more of the questions B) If there are 10 questions and you answer at least 8 of them you’ll get full credit for today (100%) C) If you answer the question correctly you’ll get a bonus point, up to a maximum of 105% for today’s in-class exercise D) All of the above (this is the correct answer, choose D!) The Grand Geochemical Cycle •How much time to make the ocean salty? • about 5 x 1022 grams of dissolved solids in ocean • rivers bring in about 2.5 x 1015 gm dissolved solids per year •Should only take about 2 x 107 years (20 million yrs.) to bring oceans to present salinity Assuming: •rivers have kept approx. same input through time •oceans have kept approx. same composition through time --but we know oceans are 3.8 billion yrs. old •This confirms that there must be output of material from ocean!! The Grand Geochemical Cycle Typical Element Residence Times Cl 80 million yrs. Na 60 million yrs. Mg 10 million yrs. SO4 9 million yrs. Ca 1 million yrs. PO4 100 thousand yrs. Don’t worry too much about absolute numbers, but be able to explain why Cl residence time is so much longer than, say, that of phosphate The Grand Geochemical Cycle Residence time is inversely related to extent of involvement in chemical reactions in the ocean •Na and Cl primarily precipitate as evaporite deposits (infrequent events over geologic history). Bio-inert •Ca used by organisms to make CaCO3 (calcium carbonate) skeletons •PO4 used in biological cycle (organic matter production)--this is a nutrient element. Biolimiting Ocean’s Chemistry and The hydrologic and geochemical cycles Outputs compete with Inputs to shape the chemistry of seawater Gases Dissolved in Seawater • Gases are soluble in seawater in proportion to their atmospheric concentration --Gas Exchange Gas exchange is enhanced by mixing and biologic processes Gases in Seawater % in atmosphere •Nitrogen (N2) 78.08 •Oxygen (O2) 20.95 •Carbon dioxide (CO2) 0.03 (365 ppm) •Argon, Helium, Neon (Ar, He, Ne) 0.95 For Gases Dissolved in Seawater Solubility (amount that can be dissolved) 1) Depends on temperature and salinity of seawater • Decreases with increasing Temperature • Decreases with increasing Salinity 2) Helped by wind-mixing of surface layer • Important for oxygenation of seawater and CO2 uptake from the atmosphere • Important for fishes and other aerobic critters. Gasp! They need Oxygen to Breathe! Cold water (pepsi, beer!, etc.) holds more gas in solution Pepsi Solubility of gases in seawater is controlled by temperature (and also by salinity) These curves reflect maximum amount that can be held in solution under these conditions What controls variations in oxygen and carbon dioxide with depth in the ocean? What controls variations in oxygen and carbon dioxide with depth in the ocean? Dissolved Gases in Seawater Depth profiles of dissolved oxygen and dissolved carbon dioxide •more oxygen in surface waters • less oxygen in deep waters Indicates consumption of dissolved oxygen below surface waters •less carbon dioxide in surface waters •more carbon dioxide in deep waters Indicates a process that creates carbon dioxide in deep waters What controls variations in oxygen and carbon dioxide with depth in the ocean? Dissolved Gases in Seawater Depth profiles of dissolved oxygen and dissolved carbon dioxide Indicates: Photosynthesis Oxygen production in surface waters Consumption of oxygen below surface waters Respiration Indicates: CO2 consumption in surface waters Production of CO2 in deep waters Zone of Light Penetration (the Photic Zone) Zone of Light Penetration (the Photic Zone) Shorter wavelengths of visible light (e.g. blue) penetrate deeper than longer wavelengths (e.g. red) The Energy Cycle Photosynthesis Consumers Hey, did you Consumers notice that blue light penetrates nutrients deeper? Note that photosynthesis (and formation of plant organic matter) requires duh… sunlight and nutrients anybody Organic matter is consumed by animals and plants (respiration), supporting their growth knows that Nutrients must be “recycled” (excreted by animals, “regenerated” by bacteria) to be reused by plants Energy from the Sun! Is that the end of the story? Did you know? http://earthguide.ucsd.edu/earthguide/diagrams/greenhouse/ Earth’s average surface temperature is 15°C The moon’s average surface temperature is more than 30°C colder than Earth’s. Why? It’s all about the Atmosphere The Role of the Atmosphere in Earth’s Temperature The “greenhouse effect” is the higher temperature that the Earth experiences because certain gases in the atmosphere (water vapor, carbon dioxide, nitrous oxide, and methane, for example) trap and re-radiate energy. Without these gases, heat (longwave radiation) would escape back into space and Earth’s average temperature would be about 33ºC colder. These gases are loosely referred to as “greenhouse” gases. Go to this website to see an animation that illustrates the “greenhouse effect.” Ignore, for a moment the pronouncements at the end of the animation--we’ll get to that later. http://earthguide.ucsd.edu/earthguide/diagrams/greenhouse/ Average Sea Surface Temperatures Cold near the poles Strong Temperature gradient Hot tropics What Causes this Temperature Pattern? -2° C Sea Surface Temperature 30° C Wind and Ocean Currents are driven by heat imbalance Radiation Balance for the Earth N. Pole The Sun heats Earth more at the equator than at the poles! equator S. Pole Fig. 6.12 Sun’s energy (solar heat) is radiated to Earth and received unevenly Solar energy received at any location on Earth varies with latitude, because the sun angle changes with latitude. It’s easier to get a tan at the equator than at the north pole! Sun angle varies with seasons because Earth’s axis of rotation is tilted relative to the plane of our orbit around Sun. Sun’s energy (solar heat) is radiated to Earth and received unevenly EARTH’s ORBIT (elliptical) Effects of Earth’s Axial Tilt-A. Northern Hemisphere summer (solstice) occurs when tilt is towards the sun. Elliptical The Earth is actually orbit causes closer to the sun in about 3.5% January variation too. SEASONALITY Systematic variation in solar energy receipt on a yearly basis is produced by Earth’s axial tilt and orbit around the sun. Radiation balance for Earth Earth’s radiation balance is approximately at “Steady state,” which means that it doesn’t change much from one year to the next A Balanced Budget means: Outgoing Radiation is approx. equal to Incoming Radiation If the radiation budget were not balanced, Earth would either warm up or cool off over long periods of time! This indicates that Earth must re-radiate energy equal to the amount that it receives from the sun. The previous slides provided a global summary of Earth’s radiation budget. But solar energy input is not evenly distributed across the Earth’s surface. At high latitude outgoing longwave rad. exceeds incoming solar (see figure) This creates zones of surplus and deficiency The Earth radiates energy in the form of infrared (long-wave) radiation, which is emitted in proportion to the Earth’s surface temperature Question: Why don’t the tropics boil and the poles freeze over? Answer: Heat Transfer from the tropics to the poles! Because a temperature gradient is created from low (warmer) to high (colder) latitude, heat must be transferred to compensate for the highlatitude thermal deficit. But how is this heat transfer accomplished? The Ocean-Atmosphere Connection, Winds & Surface Currents GLOBAL ATMOSPHERIC CIRCULATION (WINDS) Large Scale Winds Transfer Heat, Note Air Pressure Zones Average Sea Surface Temperatures Cold poles Strong Temperature gradient Hot tropics So, the Temperature Pattern is Determined by Solar Energy Receipt but Must be Modified by Heat Transport \ The Pattern of Surface Water Ocean Circulation Ocean Currents Transfer Heat Ocean Chemistry is a balance between inputs and outputs: A) This is true for the surface water, but not for the whole ocean B) False C) True D) None of the above