Chemical Kinetics - Winona State University
advertisement

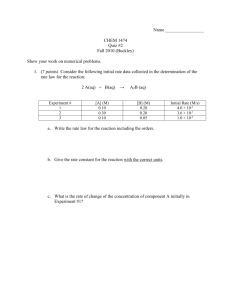
BLB 11th Chapter 14 1. 2. 3. Will the reaction occur? Ch. 5, 19 How fast will the reaction occur? Ch. 14 How far will the reaction proceed? Ch. 15 Review: concentration units, graphing (line, slope) Work with reaction rates. Determine a rate law for a given reaction from experimental data. Work with rate laws. Understand the factors that affect the rate of a chemical reaction. Calculate the activation energy. C(s, diamond) ΔH °rxn = Is the reaction favorable? C(s, graphite) studies the rates at which chemical reactions occur. gives information about how the reaction occurs, that is, the reaction mechanism Determining the reaction mechanism is the overall goal of kinetic studies. (sect. 6) 1. 2. 3. 4. 5. Physical state of the reactants – states that promote contact have faster rates; homogeneous vs. heterogeneous Concentration of the reactants: conc. ↑, rate ↑ (or pressure for gases) Temperature: temp. ↑, rate ↑ due to higher molecular energy and speed (section 5) Catalysts: rate ↑ by changing the mechanism and reaction energy (section 7) Other physical things like stirring and grinding solid reactants. Rate – change in some variable per unit time 1 rate time Reaction rate – change in concentration (M), moles, or pressure per unit time; M/s or M·s-1 Rates are determined by monitoring concentration as a function of time. Rates are positive quantities; for reactant A: [ A] rate final initial t In this reaction, the concentration of butyl chloride, C4H9Cl, was measured at various times. Rates change over time: ◦ reactant rates decrease ◦ product rates increase [ A] rate t Instantaneous rate – rate at a specific time Average rate – Δ[A] over a specific time interval Initial rate – instantaneous rate at t = 0 Note: Rates and rate laws are not based on stoichiometry!! They must be determined experimentally. The molar ratios between reactants and products correspond to the relative rates of the reaction. Relative rates – relationship between rates of reactant disappearance and product appearance at a given time. 2 HI(g) → H2(g) + I2(g) 1 [ HI ] [ H 2 ] [ I 2 ] rate 2 t t t t=0 0.1000 t=50 0.0905 Δ= t=0 0 t=50 ? Δ= 2 NO2(g) → 2 NO(g) + O2(g) Rate = 2.4 x 10-5 M/s Rate = 8.6 x 10-5 M/s Rate = 4.3 x 10-5 M/s 1. Differential rate law or rate law (section 3) 2. Shows how the initial reaction rate changes with starting concentrations Must be specific in how defined (temp., etc.) Initial rates used to determine reactant order Integrated rate law (section 4) Shows how concentration changes with time Graphical determination of the reaction order This relationship is summarized as a rate law. rate = k[NH4+][NO2-] aA + bB → cC + dD General form of rate law: [A], [B] – conc. in M or P rate = k[A]m[B]n k – rate constant; units vary m, n – reaction orders Reaction orders and, thus, rate laws must be determined EXPERIMENTALLY!!! Note: m ≠ a and n ≠ b Overall reaction order = sum of individual orders Rate constant is independent of concentration. Order (m) Δ[A] by a factor of: Effect on rate Zero (0) 2, 4, 15, ½, etc. None 2 2X 3 3X 2 4X 3 9X ½ ¼X 1st (1) 2nd (2) 1. 2. 3. 4. 5. 6. What is the order with respect to NO? What is the order with respect to H2? What is the overall order? If [NO] is doubled, what is the effect on the reaction rate? If [H2] is halved, what is the effect on the reaction rate? What are the units of k? 1. 2. Calculate the rate of reaction when the concentration of PtCl2(NH3)2 is 0.020M. What is the rate of Cl¯ production under these conditions? (b) Calculate rate when [NO] = 0.035 M and [H2] = 0.015 M. Initial Rates Method 1. Find two experiments in which all but one reactant’s concentration is constant. 2. Observe the relationship between concentration change and rate change to determine the order for that reactant. 3. Repeat for other reactant(s). See samples on pp. 568-9. Exp. # 1 [NH4+]o 0.100 [NO2¯]o 0.0050 Initial Rate (M·s-1) 1.35 x 10-7 2 0.100 0.0100 2.70 x 10-7 3 0.200 0.0100 5.40 x 10-7 Determine the rate law and calculate k. Exp. # 1 [NO]o 0.10 [Cl2]o 0.10 Initial Rate (M·min-1) 0.18 2 0.10 0.20 0.36 3 0.20 0.20 1.45 Determine the rate law and calculate k. Exp. # [BrO3¯]o 1 0.10 2 0.20 3 4 0.20 0.10 [Br¯]o 0.10 0.10 [H+]o 0.10 0.10 Initial Rate (M·s-1) 8.0 x 10-4 1.6 x 10-3 0.20 0.10 0.10 0.20 3.2 x 10-3 3.2 x 10-3 Determine the rate law and calculate k. 1st order integrate [ A] rate k[ A] t ln[ A]t kt ln[ A]o y = mx + b Plot: ln[A] vs. t slope = − k [ A]o kt, for[ A]t [ A]o ln [ A]t 2nd [ A] 2 rate k[ A] t order integrate 1 1 kt [ A]t [ A]o y = mx + b Plot: 1/[A] vs. t slope = k 1 1 kt, for[ A]t [ A]o [ A]t [ A]o Zero-order integrate [ A] 0 rate k[ A] k t [ A]t kt [ A]o y = mx + b Plot: [A] vs. t slope = − k [ A]o [ A]t kt, for[ A]t [ A]o Order Rate law zero 1st 2nd rate = k rate = k[A] rate = k[A]2 Integrated [A]t=−kt+[A]0 ln[A]t=−kt+ln[A]0 1/[A]t=kt+1/[A]0 rate law Straight[A] vs. t ln[A] vs. t 1/[A] vs. t line plot Slope −k −k k Half-life (t1/2) [A]o/2k 0.693/k 1/k[A]0 Pressure Time (s) CH3NC (torr) 0 502 2,000 335 5,000 180 8,000 95.5 12,000 41.7 15,000 22.4 To Excel for analysis Time (min) [C12H22O11] 0 0.316 39 0.274 80 0.238 140 0.190 210 0.146 To Excel for analysis t1/2 – time required for the concentration of a reactant to decrease by half of its initial value [ A]0 [ A]t 1 [ A]t @ t1/ 2 2 [ A]0 2 1st order half-life ◦ All half-lives same length of time ◦ Independent of initial concentration [ A]0 kt ln [ A]t ln 2 kt1/ 2 0.693 kt1/ 2 Note: Radioactive decay follows 1st order kinetics. t1/ 2 0.693 k 1st order reaction 2nd order half-life ◦ All half-lives different length of time ◦ Dependent on initial concentration t1/ 2 1 k [ A]0 t1/ 2 [ A]0 2k Zero half-life ◦ All half-lives different length of time ◦ Dependent on initial concentration Order Rate law zero 1st 2nd rate = k rate = k[A] rate = k[A]2 Integrated [A]t=−kt+[A]0 ln[A]t=−kt+ln[A]0 1/[A]t=kt+1/[A]0 rate law Straight[A] vs. t ln[A] vs. t 1/[A] vs. t line plot Slope −k −k k Half-life (t1/2) [A]o/2k 0.693/k 1/k[A]0 BLB 45 For the reaction A → B, k = 4.15x10-3 M-1·s-1. If the initial concentration of A is 0.100 M, how many seconds will it take for the concentration to decrease to 0.0250? Generally, as temperature increases, so does reaction rate. This is because k is temperature dependent. Collision theory In a chemical reaction, bonds are broken and new bonds are formed. In order for molecules to react, they must collide. Collisions are either effective or ineffective due to orientation of molecules. Collisions must have enough energy to overcome the barrier to reaction, the activation energy. Temperature affects the number of collisions. Molecular Collisions Cl + NOCl → NO + Cl2 Energy barrier (hump) that must be overcome for a chemical reaction to proceed Activated complex or transition state – arrangement of atoms at the top of the barrier Energy difference between the reactant and the highest energy along the reaction pathway Reaction specific Rate of reaction is dependent upon the magnitude of Ea; Ea ↓, rate ↑ (generally) Temperature independent Maxwell-Boltzman Distribution At higher T, more molecules will have adequate energy to react. This increases the reaction rate. f e Ea RT Svante Arrhenius developed an equation for the mathematical relationship between k and Ea. Ea RT k Ae A is the frequency factor, which represents the number of effective collisions. k Ae Ea RT Ea ln k ln A RT Ea 1 ln k ln A R T y = m x + b Ea m Ea m R R where : R 8.3145 molJK T in K k2 ln k1 y ln k 2 ln k1 m or x 1 1 1 1 T2 T1 T2 T1 T(°C) k (M-1s-1) 15 0.0521 25 0.101 35 0.184 45 0.332 To Excel for analysis Reactions occur in a series of elementary steps collectively called a mechanism. Determining the reaction mechanism is the overall goal of kinetic studies. One step, the rate-determining step, is much slower than the others. Usually, an intermediate (isolable) or a transition state (non-isolable) is formed at some point during the reaction. molecularity – the number of molecules that participate in a reaction Molecularity is the number of molecules reacting. Catalyst – increases the rate of a reaction without being consumed or changing chemically Accomplished by lowering the activation energy and changing the reaction mechanism. Heterogeneous vs. homogeneous catalysis Examples: ◦ Catalytic converter (p. 592) ◦ Enzymes in the body (pp. 591-3) ◦ Ozone depletion Heterogeneous catalytic ethylene hydrogenation: C2H4 + H2 → C2H6