MembraneLipidsFIN11
advertisement

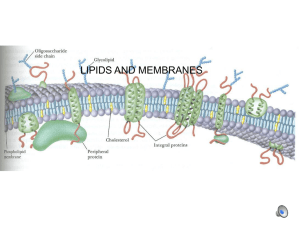
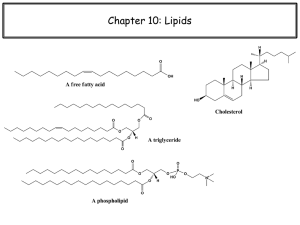
December 6, 2011 Lecturer: Eileen M. Lafer MEMBRANE LIPIDS I and II: GLYCEROPHOSPHOLIPIDS AND SPHINGOLIPIDS Reading: Stryer Edition 6: Chapter 26 OBJECTIVES: 1. Know the general structures of the principle classes of storage and membrane lipids. 2. Recognize the structures of the major glycerophospholipids (phosphatidic acid, phosphatidyl serine, phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl inositol, cardiolipin). 3. Understand the chemical differences between -ester linked and -ether linked phospholipids. 4. Recognize the structures of the major sphingolipids: ceramide, sphingomyelin, glucosylcerebroside, lactosylceramide, ganglioside GM2. 5. Understand the role that sphingolipids play as blood group determinants. 6. Know the pathways for the degradation of phospholipids and sphingolipids. 7. Know the genetic defects that lead to the following diseases: Generalized gangliosidosis, Tay-Sachs disease, Fabry's disease, Sandhoff's disease, Gaucher's disease, and Niemann-Pick disease. 8. Understand how lipids can act as signaling molecules in cells. 9. Know the mechanism of action of the non-steroidal anti-inflammatory drugs. 10. Know how phosphatidate is synthesized. 11. Understand both pathways of glycerophospholipid synthesis: a. Via CDP-diacylglycerol intermediate (PI, PG, CL) b. Via CDP-head group intermediate (PC, PE) 12. Understand the pathways that involve the modification of phosphatidylethanolamine. 13. Understand the cause of respiratory distress syndrome. 14. Know how sphingomyelin is synthesized. THE PRINCIPLE CLASSES OF STORAGE AND MEMBRANE LIPIDS All these lipids have either glycerol or sphingosine as the backbone. A third class of membrane lipids, the sterols, are discussed separately. L-GLYCEROL 3-PHOSPHATE: The backbone of phospholipids This compound can also be called D-glycerol 1-phosphate. Glycerol is pro-chiral, it has no asymetric carbons, but attachment of phosphate to either end makes it chiral. GLYCEROPHOSPHOLIPIDS 1. The common glycerophospholipids are diacylglycerols linked to head-group alcohols through a phosphodiester bond. GLYCEROPHOSPHOLIPIDS Phosphatidic acid, a phosphomonoester, is the parent compound (X=H). GLYCEROPHOSPHOLIPIDS Each derivative is named for the head-group alcohol (X) with the pre-fix "phosphatidyl-". Each derivative is named for the headgroup alcohol (X) with the pre-fix "phosphati dyl-". In caridiolipin, two phosphatidic acids share a single glycerol, hence it is also called diphosphatidly-glycerol. GLYCEROPHOSPHOLIPIDS 2. The fatty acids can vary greatly between organisms, tissues and cells. In general, they contain a saturated C16 or C18 fatty acid at C1 and a C18 to C20 unsaturated fatty acid at C2. GLYCEROPHOSPHOLIPIDS 3. Common components of cell membranes. STRUCTURES OF SOME COMMON GLYCEROPHOSPHOLIPIDS (lecithin) SOME PHOSPHOLIPIDS HAVE ETHER LINKED FATTY ACIDS 1. Plasmalogens have an ether-linked alkenyl chain where most glycerophospholipids have an ester-linked fatty acid. The head group alcohol is choline. ~50% of the heart phospholipids are plasmalogens. ETHER ESTER 2. Platelet-activating factor has a long ether-linked alkyl chain at C1. Acetic acid is ester-linked at C2, which makes it more water soluble than most glycerophospholipids. The head-group alcohol is choline. Platelet-activating factor is a potent molecular signal released from leucocytes that stimulates platelet aggregation and serotonin release. It also has a variety of effects on many tissues including roles in inflammation and the allergic response. THE PRINCIPLE CLASSES OF STORAGE AND MEMBRANE LIPIDS All these lipids have either glycerol or sphingosine as the backbone. A third class of membrane lipids, the sterols, are discussed separately. SPHINGOLIPIDS The 3-carbon backbone is analogous to the 3-carbons of glycerol. At C3 there is the long chain amino alcohol sphingosine. SPHINGOLIPIDS At C2 there is a fatty acid which is usually saturated or monounsaturated, and can be either 16,18, 22, or 24 carbons long. SPHINGOLIPIDS Ceramide is the parent compound. Other polar head groups can be attached at position X. SPHINGOLIPIDS Glycosphingolipids are a sub-group of sphingolipids that contain sachharide headgroups SIMILARITIES BETWEEN PHOSPHATIDYL CHOLINE (A GLYCEROPHOSPHOLID) AND SPHINGOMYELIN (A SPHINGOLIPID) choline choline Prominent in myelin sheath that surrounds and insulates neurons SPHINGOLIPIDS AT CELL SURFACES ARE SITES OF BIOLOGICAL RECOGNITION 1. In humans at least 60 different sphingolipids have been identified. 2. Very prominent in neuronal plasma membranes. 3. Carbohydrate moieties of sphingolipids define the human blood groups. 4. The kinds and amounts of gangliosides vary dramatically during development. GLYCOSPHINGOLIPIDS AS DETERMINANTS OF BLOOD GROUPS The human blood groups (O, A, B) are determined in part by the oligosaccharide head groups of these glycosphingolipids. Glc:D-glucose Gal:D-galactose GalNAc:N-acetyl-Dgalactosamine Fuc:fucose PHOSPHOLIPIDS AND SPHINGOLIPIDS ARE DEGRADED IN LYSOSOMES 1. For each hydrolyzable bond in a glycerophospholipid there is a specific hydrolytic enzyme in the lysosome. 2. Phospholipase A1 hydrolyzes the fatty acid at C1. 3. Phospholipase A2 hydrolyzes the fatty acid at C2. 4. When one fatty acid is removed from either C1 or C2, a lysophospholipase removes the remaining fatty acid. 5. Phospholipases C and D each split one specific phosphodiester bond in the head group. A A GLOBOSIDE LACTOSYLCERAMIDE GLUCOSYLCEREBROSIDE GANGLIOSIDES ARE DEGRADED BY A SET OF LYSOSOMAL ENZYMES A A GLOBOSIDE LACTOSYLCERAMIDE GLUCOSYLCEREBROSIDE These enzymes catalyze the stepwise removal of sugar units, finally yielding a ceramide. A A GLOBOSIDE LACTOSYLCERAMIDE GLUCOSYLCEREBROSIDE A genetic defect in any one of these hydrolytic enzymes leads to the accumulation of gangliosides in the cell, with severe medical consequences. A A GLOBOSIDE LACTOSYLCERAMIDE GLUCOSYLCEREBROSIDE Genetic counseling can predict and avert many of these diseases. NIEMANN-PICK DISEASE Defect in Sphingomyelinase Sphingomyelin accumulates in the brain, spleen, and liver Evident in infants. Causes mental retardation and early death. NIEMANN-PICK DISEASE TAY-SACHS DISEASE Defect in Hexoaminadase TAY-SACHS DISEASE Ganglioside GM2 accumulates in the brain and spleen TAY-SACHS DISEASE Electron Micrograph from a portion of a brain of an infant with Tay-Sachs disease showing abnormal ganglioside deposits in the lysosomes: TAY-SACHS DISEASE Progressive retardation in development, paralysis, blindness, and death by the age of 3 or 4 years. TAY-SACHS DISEASE SUMMARY OF DEFECTS IN GANGLIOSIDE CATABOLISM THAT CONTRIBUTE TO HUMAN DISEASE Medical Condition Defective Enzyme Major Accumulating Metabolite Generalized Gangliosidosis Beta-Galactosidase GM1 Tay-Sachs Disease Hexosaminidase A GM2 Gaucher’s Disease Glucocerebrosidase Glucosylcerebroside Niemann-Pick Disease Sphingomyelinase Sphingomyelin Sandhoff’s Disease Hexosaminidase A&B A Globoside* Fabry’s Disease Alpha-Galactosidase A A Globoside* *The globosides that are substrates for the defective enzymes in these diseases do not have specific names, but are referred to by the broad term globoside. LIPIDS AS SIGNALS : 1. Phosphatidyl Inositols and its phosphorylated derivatives act to regulate cell structure and metabolism. 2. Eicosanoids are paracrine hormones derived from arachidonic acid-containing membrane phospholipids. They are involved in reproductive function, inflammation, fever, pain, blood clot formation, blood pressure regulation, gastric acid secretion, and other biological processes. PHOSPHATIDYLINOSITOLS PIP2 is hydrolyzed by phospholipase C in response to hormonal signals. Both of the metabolites produced act as intracellular messengers. ARACHIDONIC ACID AND SOME EICOSANOID DERIVATIVES In response to hormonal signals, phospholipase A2 cleaves arachadonic-containing membrane lipids to release arachidonic acid. Arachidonic acid can then serve as a precursor for several eicosanoids including prostaglandin E1, thromboxane A1 and leukotriene A4: Non-steroidal anti-inflammatory drugs (NSAIDS) such as aspirin, naproxen, and ibuprofen block the formation of prostaglandins and thromboxanes from arachidonate by inhibiting the enzyme cyclooxygenase (prostaglandin H2 synthase). BIOSYNTHESIS OF MEMBRANE PHOSPHOLIPIDS 1. Synthesis of the backbone molecule (glycerol or sphingosine). 2. Attachment of fatty acids to the backbone in ester or amide linkage. 3. Addition of a hydrophilic head group joined to the backbone through a phosphodiester linkage. 4. In some cases, alteration or exchange of the head group to yield the final phospholipid product. TRIACYLGLYCEROLS AND GLYCEROPHOSPHOLIPIDS ARE SYNTHESIZED FROM THE SAME PRECURSORS (1) Formation of Glycerol-3-phosphate: acyl groups (2) Fatty are activated by formation of fatty acyl-CoA. Then they are transferred to ester linkage with glycerol-3phosphate. PHOSPHATIDIC ACID = DIACYLGLYCEROL 3-PHOSPHATE THERE ARE TWO STRATEGIES FOR ATTACHING HEAD GROUPS Diacylglcerol activated with CDP: Phosphatidylinositol Cardiolipin Phosphatidylglycerol Headgroup activated with CDP: Phosphatidylcholine Phosphatidylethanolamine PHOSPHATIDIC ACID IS THE PRECURSOR OF BOTH TRIACYLGLYCEROL AND GLYCEROPHOSPHOLIPIDS MODIFICATION PATHWAYS: Phosphatidylserine and phosphatidylethanolamine can be interconverted by a reversible head group exchange reaction. MODIFICATION PATHWAYS: In mammals, phosphatidylserine is synthesized from phosphatidylethanolamine by this mechanism. MODIFICATION PATHWAYS: This reaction is catalyzed by phosphatidylethanolamineserine transferase. Phosphatidylethanolamine may also be converted to phosphatidylcholine (lecithin) by the addition of three methyl groups to its amino group. S-adenothylmethione is the methyl donor. This reaction is catalyzed by a methyltransferase. The multiple pathways of phospholipid biosynthesis reflect the importance of phospholipids in membrane structure and function. RESPIRATORY DISTRESS SYNDROME: Failure to synthesize enough dipalmitoyl phosphatidylcholine which is an important component of lung surfactant. It functions to reduce the surface tension of the fluid that surrounds the alveoli of the lung to prevent lung collapse at the end of the expiration phase of breathing. Premature infants may suffer from respiratory distress syndrome because their immature lungs do not synthesize enough dipalmitoyl phosphatidyl choline. SPHINGOLIPID BIOSYNTHESIS I 1. Synthesis of 18 carbon sphinganine from palmitoylCoA and serine. SPHINGOLIPID BIOSYNTHESIS I 2. Attachment of a fatty acid in amide linkage to yield N-acylsphinganine. SPHINGOLIPID BIOSYNTHESIS II 3. Desaturation of the sphinganine moiety to form N-acylsphingosine. SPHINGOLIPID BIOSYNTHESIS II 4. Attachment of a head group to produce a sphingolipid such as a cerebroside or sphingomyelin.