Redefining the Treatment
Algorithm for Type 2
Diabetes – 2009
Robert J. Rushakoff, MD
Professor of Medicine
University of California, San Francisco
robert.rushakoff@ucsf.edu
Welcome to Today’s
Medical Education Program!
• I am pleased to be here with you on behalf of Merck &
Co., Inc. who is sponsoring this medical education
program.
• The program you are participating in is not an accredited
Continuing Medical Education program.
• The information presented throughout the program will
be consistent with FDA guidelines.
What do you think of when
told:
2-2.5 fold increased risk of CHF
2-3 fold increase in risk for initial MI
3 fold increase in risk for pancreatitis
Decreased leukocyte function
Risk for lactic acidosis
Increased risk for renal failure, retinopathy,
neuropathy
Type 2
Diabetes
Insulin
secretion
β
Hyperglycemia
hepatic
glucose output
Glucose
uptake
glucose
utilization
β
α
Postprandial
glucagon
secretion
Hyperglycemia
β
α
Hyperglycemia
FFA
Lipotoxicity
Incretins
β
α
Hyperglycemia
β
α
Hyperglycemia
Altered
glucose
reabsorption
Altered
Hypothalamic
β
α
Hyperglycemia
Appetite
Control
Ominous Octet
β
α
Hyperglycemia
Fundamental Questions
Just because a drug may work at
one or more of the sites of defect
in Type 2 DM - what about:
Efficacy
Side effects
Actually improve outcomes or
make them worse
Decrease mortality or kill people
Fundamental Questions
Is there anything wrong with the
current group of medications?
Do the newer medications fix what is
wrong with the older medications?
Does it really matter what medication is
used first, second, third?
Does it really matter what medication is
used?
New Drug Truthiness
Often no clinically relevant literature
published before medication is released
Studies performed to obtain FDA
approval are useful for FDA approval
Clinically useful studies may lag release
to market by 5 years, or are never done
Today
What are the goals?
What differentiates the medications?
Does it really matter what medication is
used?
Put it all together – ADA way, AACE
way and of course, MY WAY
Relationship Between Plasma Glucose and
HgA1c
Diabetes Care 31:1473–1478, 2008
Hemoglobin A1c
50% of level determined in
previous month
25% by month before
12.5% by month before
12.5% by month before
Hemoglobin A1c
False High Levels
Thalassemia (Hgb F)
Lead poisoning
Large amount of ASA
High alcohol, Tg
bilirubin levels
Hemoglobinopathies
J,K,I,H, Bart’s,
Raleigh, Long Island
and South Florida
False Low Levels
Hemoglobinopathies
S,D,C,E,G, Lepore
and O-Arab.
Hemolytic anemia,
bleeding
Large ingestions of
Vitamin C and E
The relationship between baseline A1C group and
observed reduction from baseline in A1C and in FPG
Baseline
A1C (%)
n enrolled in
clinical trials
Change in
A1C (%)
Change in FPG
(mmol/l)
6.0–6.9
410
–0.2
–0.5
7.0–7.9
1,620
–0.1
–0.8
8.0–8.9
5,269
–0.6
–1.6
9.0–0.9.9
1,228
–1.0
–2.3
10.0–11.8
266
–1.2
–3.4
Diabetes Care 29:2137-2139, 2006
ADVANCE: Relative Effects of Glucose-Control Strategy on
All Prespecified Primary and Secondary Outcomes
The ADVANCE Collaborative Group. N Engl J Med 2008;358:2560-2572
ACCORD: Hazard Ratios for the Primary Outcome and Death from Any
Cause in Prespecified Subgroups
The Action to Control Cardiovascular Risk in Diabetes Study Group. N Engl J Med 2008;358:2545-2559
VADT - Veterans
Administration Diabetes Trial
•1742 Enrollees
•97% male
•Mean age 60.4
•BMI 31.3
•Majority had
multiple CV risk
factors
•72% HTN
•40% macrovascular
dx
•62% retinopathy
•43% neuropathy
VADT - Veterans
Administration Diabetes Trial
Primary Endpoint: NO DIFFERENCE IN
CARDIOVASCULAR DISEASE
OUTCOMES
Standard: 29.3%
Intensive: 27.4%
(predicted – 40%)
(predicted – 31.6%)
VADT - Veterans
Administration Diabetes Trial
Baseline Predictor of CVD:
Age and prior CVD event
On-trial hypoglycemia – low glucose
and altered consciousness in the three
months prior to an event was predictive
of CVD outcome
VADT - Veterans
Administration Diabetes Trial
When duration of DM factored in:
Intensive glycemic control showed
benefit
Benefit declines until about 12-15
years of disease
UKPDS: 10 year follow-up
Glucose Control
Between-group differences in HgA1c gone after 1 year
In the sulfonylurea–insulin group, relative reductions in risk
persisted at 10 years for:
any diabetes-related end point (9%, P=0.04)
microvascular disease (24%, P=0.001)
risk reductions for myocardial infarction (15%, P=0.01)
death from any cause (13%, P=0.007)
In the metformin group:
any diabetes-related end point (21%, P=0.01)
myocardial infarction (33%, P=0.005)
and death from any cause (27%, P=0.002).
Published at www.nejm.org September 10, 2008
Effect of Metformin-Containing Antidiabetic
Regimens on All-cause Mortality in Veterans
With Type 2 Diabetes Mellitus
Decreased Hazard Ratio for all cause mortality for
patients on metformin
Increased Hazard Ratio for all cause mortality for
patients on insulin:
vs no metformin – 0.77 (p<0.01)
1.62 (p<0.001)
Decreased Hazard Ratio for all cause mortality for
patients on metformin and insulin vs insulin
0.62 (p<0.04)
Am J Med Sci 2008; 336:241-247
ADA Targets
for Glycemic Control
Biochemical Index
Preprandial plasma glucose
Peak postprandial plasma glucose
Hemoglobin A1c
Goal
80–130 mg/dl (5-7.2 mmol/l)
<180 mg/dl (<10 mmol/l)
<7 (%)
ADA Targets
for Glycemic Control
Key concepts in setting glycemic goals: A1C is the primary target for glycemic control.
Goals should be individualized based on:
duration of diabetes
age/life expectancy
comorbid conditions
known CVD or advanced microvascular complications
hypoglycemia unawareness
individual patient considerations
More or less stringent glycemic goals may be appropriate for individual patients.
Postprandial glucose may be targeted if A1C goals are not met despite reaching
preprandial glucose goals.
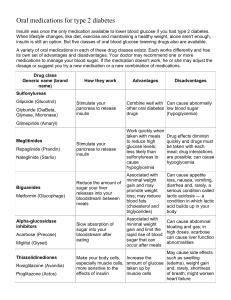
Class
Generic Name
(Brand Name)
Mechanism of
Action
Dosage
Sulfonylureas
Glyburide
(Micronase)
Glipizide
(Glucotrol)
glimepiride
(Amaryl)
Relative
Effective
-ness
Major Side Effects /
Interactions / Uses
Cost
Weight
Effects
(average)
1
2.5-10 mg bid
Stimulate
insulin release
from beta cells 5-20 mg bid
of the
pancreas
0.5-4 mg qd
1
1
Hypoglycemia
Gain
2 lbs
$
Class
Generic Name
(Brand Name)
Mechanism of
Action
Dosage
Relative
Effective
-ness
Major Side Effects /
Interactions / Uses
Cost
Weight
Effects
(average)
Stimulate insulin
release
1
Hypoglycemia
Meglitinides Stimulate
0.5-2 mg tid
insulin
(before
release from meals)
repaglinide
beta cells of
(Prandin)
the pancreas
60-360 mg
nateglinide
tid
(Starlix)
(before
meals)
1
Hypoglycemia
Useful in pts on
glucocorticoids and in pts
Gain 1 lb $$$
with renal failure who
often have good FBS and
high BS over the course
of the day
Prandin is short-acting.
Starlix is very short-acting
Sulfonylureas
.8
Gain
2 lbs
$
Class
Generic Name
(Brand Name)
Sulfonylureas
Meglitinides
Mechanism of
Action
Dosage
Stimulate insulin
release
Stimulate insulin
Biguanide
Primarily
500-2000
metformin
inhibits
mg daily
(Glucophage) hepatic
with meals
gluconeogen
-esis.
Relative
Effective
-ness
Major Side Effects /
Interactions / Uses
1
Hypoglycemia
.8-1
Hypoglycemia
short-acting
1
Diarrhea, nausea,
vomiting
Increased risk of lactic
acidosis if impaired renal
or hepatic function or
heavy EtOH use
Cost
Weight
Effects
(average)
Gain
2 lbs
$
Gain 1 lb
$$$
Loss
2-3 lbs
$
Metformin and Lactic Acidosis
• “Metformin may provoke lactic Acidosis which is
most likely to occur in patients with renal
impairment. It should not be used with even
mild renal impairment” 1
• Metformin probably not as unsafe as previously
thought.
– 25% users have relative contraindication 2
– Patient’s with lactic acidosis usually have
acute renal failure 3
1.
2.
3.
Joint Formulary Committee British National Formulary. 2006:353
Diabet Med 2001; 18:483-488
Diabet Med 2007; 24:494-497
Metformin and eGFR
• 186 x (Creat / 88.4)-1.154 x (Age)-0.203 x (0.742 if
female) x (1.210 if black)
• Current Guidelines call for discontinuation of
Metformin serum creatinine >150 umol/l (1.7
mg/dl).
• Estimated GFR (eGFR) being introduced as
possible better measure of renal function than
serum creatinine alone
• eGFR of 36 ml/min per 1.73m2 would
be somewhat neutral to current use
Class
Generic Name
(Brand Name)
Sulfonylureas
Meglitinides
Mechanism of
Action
Dosage
Stimulate insulin
release
Stimulate insulin
Biguanide
inhibits hepatic
gluconeogenesis.
Alphaglucosidase
Inhibitor
acarbose
(Precose)
Inhibits
enzymes
needed to
break down
complex
CHO in the
small
intestine
50 mg with
1st bite of
each meal
(start at
12.5 mg and
titrate up
over weeks)
Relative
Effective
-ness
Major Side Effects /
Interactions / Uses
1
Hypoglycemia
.8-1
1
0.7
Cost
Weight
Effects
(average)
Gain
2 lbs
$
Hypoglycemia
short-acting
Gain 1 lb
$$$
Diarrhea
lactic acidosis
Loss
2-3 lbs
$
Gas/ GI upset
Loss
1-2 lbs
$$$
Treatment of Hypoglycemia in
Patients Treated with Acarbose
• In case of hypoglycemia
(due to sulfonylurea or insulin treatment)
– Glucose (dextrose) must be administered
– Sucrose and complex carbohydrates should
not be administered
Bile Acid Sequestrants
• Bile acid sequestrants lower LDL cholesterol
• Colesevelam (Welchol) a bile acid sequestrant, lowers
glucose levels and AIC levels in T2D patients
Thiazolidenediones
–The bad
–The good
–The very ugly
TZDs and Liver Disease
• From troglitazone – contraindicated in
patients with liver disease
• Diabetes patients frequently have fatty
liver (NASH---Non- Alcoholic Steatorrhoeic
Hepatosis) with elevated LFT
• TZDs decrease liver fat and improve
NASH
• TZDs may be best treatment for NASH
and preventing cirrhosis
Rushakoff RJ: Normalization of abnormal liver function tests in Type 2 diabetic
patients after administration of Troglitazone. Diabetes 48 supplement 1999
Current TZD Side Effects
• Weight Gain: 5-12 lbs in 1 year
– Blunted with metformin
– Worse with insulin
• Edema: 4-30%
– Unresponsive to diuretics
• BUT:
– Increased Cardiac Index
– Increased Stroke volume
– Decreased systemic resistance
– Decreased Blood Pressure
Thiazolidinediones and Risk of Repeat Target
Vessel Revascularization Following Percutaneous
Coronary Intervention
Diabetes Care 30:384-388, 2007
Positive Side to TZDs
•
•
•
•
•
Reduction in glucose
Reduces BP
Reduces albuminuria
Reduces CRP
Possible DM
prevention
• Reduces NASH
• Reduces LFT
• Reduces IMT
• Reduces stent failure
• Reduces death after
CHF
• Increases adiponectin
• Increases HDL
NEW ENGLAND
JOURNAL of MEDICINE
The
ESTABLISHED IN 1812
JUNE 14, 2007
VOL. 356
NO. 24
Effect of Rosiglitazone on the Risk of Myocardial Infarction
And Death from Cardiovascular Causes
Steven E. Nissen, M.D., and Kathy Wolski, M.P.H.
CONCLUSIONS
Rosiglitazone was associated with a significant increase in the risk of myocardial
infarction and with an increase in the risk of death…that had borderline significance.
Meta-analysis of MI and Death risk
with rosiglitazone
n = 15,560 on rosiglitazone; n = 12,283 on comparator drug or placebo
Rosiglitazone
group
Study
Control
group
No. of events/Total no. (%)
Odds ratio
(95% CI)
P
Myocardial infarction
Small trials combined
44/10,280 (0.43)
22/6105 (0.36)
1.45 (0.88–2.39)
0.15
DREAM
15/2635 (0.57)
9/2634 (0.34)
1.65 (0.74–3.68)
0.22
ADOPT
27/1456 (1.85)
41/2895 (1.44)
1.33 (0.80–2.21)
0.27
Overall
86
1.43 (1.03–1.98)
0.03
72
86/14371 (.60%) 72/11634 (0.62%)
Relative Risk = 86/72 = 1.19
Absolute Risk = -.02%
Nissen SE, Wolski K. N Engl J Med. 2007;356.
Comparison of RSG to SU or MET
MI/CV Death/Stroke
Meta-analysis database (ICT), ADOPT and RECORD
Rosiglitazone and Cardiovascular Events
Meta-Analytic Subgroups
Myocardial Infarction
Uncorrected (Peto)
Corrected (MH/CC)
1.45 (0.88-2.39)
Small trials combined
(N=16391)
1.16 (0.76-1.78)
DREAM (N=5269)
ADOPT (N=4351)
Overall pooled data
(N=26011)
1.43 (1.03-1.98)
0
1
2
3
Odds ratio
1.28 (0.95-1.72)
4 0
1
2
3
Odds ratio
4
Center for Drug Evaluation and Research
Joint Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee and the
Drug Safety and Risk Management Advisory Committee
July 30, 2007
David Graham, MD MPH
Center for Drug Evaluation and Research
Joint Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee and the
Drug Safety and Risk Management Advisory Committee
July 30, 2007
David Graham, MD MPH
PANIC
Rosiglitazone Associated Fractures in
Type 2 Diabetes: An Analysis From
ADOPT
Diabetes Care Publish Ahead of Print, published online on February 5, 2008
Changes in BMD during pioglitazone or
placebo treatment in patients with PCOS
J Clin Endocrinol Metab. 2008 Feb 19 [Epub ahead of print]
Class
Generic Name
(Brand Name)
Mechanism of
Action
Dosage
Relative
Effective
-ness
Major Side Effects /
Interactions / Uses
Stimulate insulin
release
Stimulate insulin
1
Hypoglycemia
1
Biguanide
inhibits hepatic
gluconeogenesis.
1
Alpha-glucosidase
Inhibitor
Decreased CHO
absorption
Sulfonylureas
Meglitinides
Thiazolidine Insulin
-diones
sensitizers—
Activate
4-8 mg daily
rosiglitazone receptor
molecules
(Avandia)
inside cell
nuclei to
15-45 mg
pioglitazone
daily
decrease
(Actos)
insulin
resistance
0.7
1
1
Cost
Weight
Effects
(average)
Gain
2 lbs
$
Hypoglycemia
short-acting
Gain 1 lb
$$$
Diarrhea
lactic acidosis
Loss
2-3 lbs
$
Gas/ GI upset
Loss
1-2 lbs
$$$
Weight gain, edema
which is resistant to
diuretic therapy, CHF.
Associated with bone loss
and fractures.
Gain
12 lbs
$$$
Comparison of metabolic effects of pioglitazone,
metformin, and glimepiride over 1 year in Japanese
patients with newly diagnosed Type 2 diabetes
114 drug naïve patients
Initial HgA1c
Duration DM about 3 years
Initial Hga1c 10%
Body mass index 25
Diabetes Medicine 2005; 22:980-985
Time course of reduction in glycated haemoglobin
(HbA1c) in patients receiving pioglitazone (O), metformin
(●), or glimepiride (). Data are mean ±sd. *P < 0.05;
**P < 0.01; ***P < 0.005 vs. baseline.
Diabetes Medicine 2005; 22:980-985
Mean Change
(mg/dl)
Fasting Plasma glucose: Mean
Change From Baseline
40
20
0
-20
-40
-60
-80
Continued
glyburide
(n=209)
Switched to
metformin
(n=210)
0
Diabetes 452:146, 1993
7
14
Weeks
21
28
Metformin +
glyburide
(n+213)
Generic Oral Hypoglycemic Slide
Change from Drug A to B, C, or D
Add Drug A to B, or B to A
HgA1c
Add Drug C
Add Drug D
Time
Weight Changes Associated with AntiHyperglycemic Therapies for Type 2 Diabetes
12
10
10.84
8
7.32
6
4
2
3.94
0
-2
-4
-6
Sulfonylurea
Metformin
Insulin
TZD
-5.29
Change in Weight
ADA Scientific Meeting 2005 ABS 13-or
Postprandial Glucose Control
Postprandial Glucose Excursions in
Subjects With or Without Diabetes
Serum Glucose Value (mg/mL)
350
Subjects
Without diabetes
Type 1 diabetes
Type 2 diabetes
300
250
200
Meal Event
150
100
50
0
–1
0
1
2
3
4
5
Time (hours)
Shin J et al. Abstract 424-P. ADA; 2004: New Orleans, La.
6
7
8
Relative Contribution of FPG and PPG to Overall
Hyperglycemia Depending on A1C Quintiles
Postprandial glucose
Fasting glucose
Contribution, %
100
80
60
40
20
0
<7.3
n=58
7.3–8.4
n=58
8.5–9.2
9.3–10.2
>10.2
n=58
n=58
n=58
A1C
Monnier L et al. Diabetes Care. 2003;26:881–885.
INCRETINS
• Gut factors that promote insulin
secretion in response to nutrients
•Major incretins: GLP-1, CCK, GIP
Oral Glucose Promotes
More Insulin Release than
IV Glucose - Indicating a
Role for Incretins
Plasma Insulin Responses to Oral and
Intravenous Glucose
Non-Diabetic
Diabetic
Insulin (U/mL)
60
30
0
Oral
Intravenous
90
Insulin (U/mL)
Oral
Intravenous
90
60
30
0
0
30
60
90
Minutes
120 150 180
0
30
60
90
120 150 180
Minutes
J Clin Invest 1967; 46:1954-1962
pmol/L
pmol/L
*
*
*
*
*
*
0
250
200
150
100
50
0
*
*
*
*
*
*
*
*
40
30
20
10
0
20
20
15
15
10
5
0
–30
*
*
*
10
*
5
Infusion
0
60
120
pmol/L
Glucagon
*
250
200
150
100
50
mU/L
Insulin
15.0
12.5
10.0
7.5
5.0
2.5
0
Placebo
mg/dL
Glucose
mmol/L
Glucose-Dependent Effects of GLP-1 on Insulin and Glucagon
Levels in Patients With Type 2 Diabetes
GLP-1
*P <0.05
Patients with type 2
diabetes (N=10)
When glucose levels
approach normal values,
insulin levels decreases.
When glucose levels
approach normal values,
glucagon levels rebound.
0
180
240
Minutes
Adapted with permission from Nauck MA et al. Diabetologia. 1993;36:741–744. Copyright © 1993 Springer-Verlag.
GLP-1 and GIP Are Degraded by the DPP-4 Enzyme
Meal
Intestinal
GIP and
GLP-1
release
GIP-(1–42)
GLP-1(7–36)
Intact
DPP-4 (Dipeptidyl
Peptidase IV)
Enzyme
Rapid Inactivation
Half-life*
GLP-1 ~ 2 minutes
GIP ~ 5 minutes
GIP and GLP-1
Actions
Deacon CF et al. Diabetes. 1995;44:1126–1131.
*Meier JJ et al. Diabetes. 2004;53:654–662.
GIP-(3–42)
GLP-1(9–36)
Metabolites
Incretin Drugs
GLP Agonists
Exenatide
Liraglutide
CJC-1131
AVE-0010
Albugon
Glp-1-transferin
Exenatide Lar
DPP IV Inhibitors
Vildagliptin
Sitagliptin
Saxagliptin
PSN-931
Takeda-Syrrx
Sitagliptin
F
F
NH2
O
N
N
F
N
N
CF3
Improvements in HbA1C With Initial Coadministration of Sitagliptin and Metformin
Mean Baseline HbA1C = 8.8%
N=1091
Placebo
HbA1C (%)*
-0.5
Sita 100 mg QD
Met 500 mg BID
-1.0
-0.8
-1.5
-2.0
-2.5
Met 1000 mg BID
-1.0
Sita 50 mg BID +
Met 500 mg BID
Sita 50 mg BID +
Met 1000 mg BID
-1.3
-1.6
-2.1
* Placebo-subtracted LS mean change form baseline at Week 24.
Sita=sitagliptin; Met=metformin.
Aschner P, et al. Oral presentation at the EASD 42nd Annual Meeting; 14-17 September 2006; Copenhagen.
Proportion of Patients Achieving HbA1C Goals
70
60
To Goal (%)
Placebo
50
Sita 100 mg QD
40
Met 500 mg BID
30
Met 1000 mg BID
20
Sita 50 mg BID
+ Met 500 mg BID
10
Sita 50 mg BID
+ Met 1000 mg BID
0
HbA1C <6.5%
HbA1C <7.0%
Sita=sitagliptin; Met=metformin.
Aschner P, et al. Oral presentation at the EASD 42nd Annual Meeting; 14-17 September 2006; Copenhagen.
Initial Combination Therapy With Sitagliptin Plus
Metformin Study: A1C Results at 104 Weeks
(Extension Study)a
79
104-week results
Mean baseline A1C = 8.5%–8.7%
LSM A1C Change From Baseline, %
0.0
n=50
n=64
n=87
n=96
n=105
Sitagliptin 100 mg qd
Metformin 500 mg bid
–0.5
Metformin 1,000 mg bid
–1.0
–1.1 b
–1.5
–2.0
Sitagliptin 50 mg bid +
metformin 500 mg bid
–1.1 b
–1.3
Sitagliptin 50 mg bid +
metformin 1,000 mg bid
–1.4
–1.7
bid=twice a day; LSM=least-squares mean; qd=once a day.
aResults include only randomized patients who agreed to enter the extension study, had not received glycemic rescue therapy through week 54, took at least 1
dose of study medication after week 54, and had at least 1 post-54-week A1C measurement.
bValues represented are rounded, actual values 1.15 for Sitagliptin 100 mg qd and 1.06 for Metformin 500 mg bid.
Data available on request from Merck & Co., Inc. Please specify 20852883(1)-JAN.
DPP-4 Study Summary
Sitagliptin vs glipizide added to metformin
52 weeks, 100 mg/d vs 20 mg/d
Baseline HgA1c 7.5
Both 0.67% reduction in HgA1c
Both about 60% reached HgA1c <7
Hypoglycemia –
• glipizide: 32%
• sitagliptin: 4.9%
Class
Generic Name
(Brand Name)
Mechanism of
Action
Dosage
Relative
Effective
-ness
Major Side Effects /
Interactions / Uses
Stimulate insulin
release
Stimulate insulin
1
Hypoglycemia
1
Biguanide
inhibits hepatic
gluconeogenesis.
1
Alpha-glucosidase
Inhibitor
Decreased CHO
absorption
Sulfonylureas
Meglitinides
Incretins
exenatide
(Byetta)
sitagliptin
(Januvia)
Mimics GLP-1
(gut hormone
which affects
insulin,
glucagon,
gastric
emptying and
satiety)
DPP-4 inhibitor
(enzyme that
breaks down
GLP-1)
Gain
2 lbs
$
Hypoglycemia
short-acting
Gain 1 lb
$$$
Diarrhea
lactic acidosis
Loss
2-3 lbs
$
Gas/ GI upset
Loss
1-2 lbs
$$$
1
Weight gain, edema, fractures
Gain
12 lbs
$$$
1
Nausea, Vomiting,
constipation, pancreatitis
(?)
Weight loss achieved
through appetite
suppression
Loss
8 lbs
$$$
1
Side effects are rare. Occ
GI side effects.
Pancreatitis (?), vasculitis
(?)
Neutral
$$$
0.7
Thiazolidinediones Insulin
5-10 mcg
bid SQ
100, 50, or
25 mg daily
(dose by Cr
Cl)
Cost
Weight
Effects
(average)
Conventional Therapies Do Not Influence b-Cell Failure:
UKPDS
Overweight
Non-Overweight
Chlorpropamide
Metformin
Insulin
Glibenclamide
ß cell function (%)
HbA1c(%)
9
8
7
cohort, median values
6
0
100
100
80
80
60
60
40
40
20
20
0
-1
0
2
4
6
8
Years from randomization
10
0
1
2
3
4
UKPDS 16: Diabetes 1995; 44: 1249-1258
6
7
0
1
2
3
4
5
6
Years from randomization
Conventional
UKPDS 34. Lancet 1998; 352: 854-865
5
Sulphonylurea
Metformin
7
0
ß cell function (%)
10 Conventional
Overweight
DIGAMI2
(European Heart J. Prepublication Feb 2005)
Group 1 – IV insulin then long term SQ insulin
Group 2 – IV insulin then standard treatment
Group 3 – Standard treatment
Mortality
Effect of different updated glucose lowering treatments on mortality and
morbidity
Mellbin, L. G. et al. Eur Heart J 2008 29:166-176
Class
Generic Name
(Brand Name)
Sulfonylureas
Meglitinides
Mechanism of
Action
Dosage
Stimulate insulin
release
Stimulate insulin
Biguanide
inhibits hepatic
gluconeogenesis.
Alpha-glucosidase
Inhibitor
Decreased CHO
absorption
.8-1
1
1
1
sitagliptin
Insulin
Hypoglycemia
1
Increase insulin,
decrease
glucagon
Titrated
to need
Major Side Effects /
Interactions / Uses
1
0.7
Thiazolidinediones Insulin
Incretins
exenatide
Relative
Effective
-ness
Cost
Weight
Effects
(average)
Gain
2 lbs
$
Hypoglycemia
short-acting
Gain 1 lb
$$$
Diarrhea
lactic acidosis
Loss
2-3 lbs
$
Gas/ GI upset
Loss
1-2 lbs
$$$
Weight gain, edema, fractures
Gain
12 lbs
$$$
Nausea
Weight loss
Loss
8 lbs
$$$
Side effects are rare.
Neutral
1+ Hypoglycemia
Gain $$
8 lbs
Drug Cost Comparison
Drug and Dose
Glucose Strips (2 per day)
Sulfonylurea
Rapaglinide 2 mg tid
Acarbose 100 mg tid
Metformin 1000 bid
Rosiglitazone 8 mg qd
Pioglitazone 45 mg/d
Sitagliptin
Exenatide
Colesevelam 3750 mg/d
Glargine, 45 U/d
24 hour fitness center
YMCA
Cost/month
$60
Generic $4-14
Brand $50
$175
$88
Generic $ 4-32
Brand $132
$223
$222
$181
5mcg $230
10mcg $255
$212
$150
$44
$60
HgA1c
Insulin
added
10
3
Drugs
9
2
Drugs
1
Drug
8
7
No
Meds
2009 ADA Type 2 Consensus Statement
Diabetes Treatment Algorithm
An American Diabetes Association consensus statement represents the authors’ collective analysis,
evaluation, and opinion at the time of publication and does not represent official association opinion.
Diabetes Care. Published online Oct 22, 2008
Road Maps to Achieve Glycemic Control
In Type 2 Diabetes Mellitus
ACE/AACE Diabetes Road Map Task Force
Chairpersons
Paul S. Jellinger, MD, MACE, Co-Chair
Jaime A. Davidson, MD, FACE, Co-Chair
Task Force Members
Lawrence Blonde, MD, FACP, FACE
Daniel Einhorn, MD, FACP, FACE
George Grunberger, MD, FACP, FACE
Yehuda Handelsman, MD, FACP, FACE
Richard Hellman, MD, FACP, FACE
Harold Lebovitz, MD, FACE
Philip Levy, MD, FACE
Victor L. Roberts, MD, MBA, FACP, FACE
© 2007 AACE. All rights reserved. No portion of the Roadmap may be altered, reproduced
or distributed in any form without the express permission of AACE.
Road Map to Achieve Glycemic Goals: Naïve to Therapy (Type 2)
Achieve ACE
Glycemic Goals†
( FPG, PPG, and A1C )
Initial
A1C%
Lifestyle
Lifestyle
Modification
7-8
Modification
6-7
Assess
FPG
and PPG
Target:
PPG
and FPG
Intervention
Initial Therapy
Preferred:
• Metformin4
• TZD10,11
• AGI
• DPP-4 Inhibitor
Alternatives
• Glinides
• SU (low dose)
• Prandial insulin5,8
Combine Therapies 6,7
Alternatives
• Metformin
• Prandial insulin5,8
• Glinides
• AGI
• Premixed insulin
• TZD
preparations8
• SU
• Basal insulin
• DPP-4 Inhibitor
analog9
†
ACE Glycemic Goals
* Available as exenatide
≤ 6.5% A1C
1 Indicated for patients not at goal despite SU and/or
metformin or TZD therapy; incretin mimetic is not
< 110 mg/dL FPG
indicated for insulin-using patients
< 110 mg/dL Preprandial
4 Preferred first agent in most patients
< 140 mg/dL 2-hr PPG
5 Rapid-acting insulin analog (available as lispro, aspart and
glulisine), inhaled insulin, or regular insulin
6 Appropriate for most patients
7 2 or more agents may be required
8 Analog preparations preferred
9 Available as glargine and detemir
10 A recent report (NEJM; 6/14/07) suggests a possible link of
rosiglitazone to cardiovascular events that requires further evaluation.
11 Cannot be used in NYHA CHF Class 3 or 4
Endocr Pract. 2007;13:260-268
Access Roadmap at:
www.aace.com/pub
Continuous
Titration
of /Rx
Monitor
( 2 - 3 months )
adjust Rx to
maximal
effective dose
to meet ACE
Glycemic
Goals
Monitor /
adjust Rx to
maximal
effective dose
to meet ACE
Glycemic
Goals
If ≤ 6.5% A1C Goal
Not Achieved
Intensify Lifestyle
Modification
Intensify or combine Rx
including incretin mimetic*1
If ≤ 6.5% A1C Goal
Not Achieved
Intensify Lifestyle
Modification
Intensify or combine Rx,
including incretin mimetic
with SU, TZD, and/or
metformin
ACE/AACE Diabetes Road Map Task Force
Paul S. Jellinger, MD, MACE, Co-Chair
Jaime A. Davidson, MD, FACE, Co-Chair
Lawrence Blonde, MD, FACP, FACE
Daniel Einhorn, MD, FACP, FACE
George Grunberger, MD, FACP, FACE
Yehuda Handelsman, MD, FACP, FACE
Richard Hellman, MD, FACP, FACE
Harold Lebovitz, MD, FACE
Philip Levy, MD, FACE
Victor L. Roberts, MD, MBA, FACP, FACE
© 2007 AACE. All rights reserved. No portion of the Roadmap may be altered,
reproduced or distributed in any form without the express permission of AACE.
TYPE 2 DIABETES
SYMPTOMATIC
NO
And very high
YES
Start on
sulfonylurea or insulin
Start Metformin
Referral for:
•Diet
•HGM
•Sick Day Rules
•Exercise (+/- EST)
•Foot Care
Goal Met
NO
YES
Continue Current Treatment
Add
Medication
Referral for:
•Diet
•HGM
•Exercise
•Foot Care
Consider
transition
to metformin
TYPE 2 DIABETES
Metformin
Thin or no injection
OBESE
Exenatide
THIN
Sulfonylurea
Goal Not
Met
Add Sulfonylurea
(consider TZD)
Goal Not
Met
Sitagliptin
(consider TZD)
Goal Not
Met
•Start insulin – use pens
•Add detemir, glargine or PM NPH (isolated fasting hyperglycemia or insurance)
•? of which existing meds to continue, generally all
•Change to bid premixed insulin
•? of which existing meds to continue, generally just metformin
•Change to basal and with premeal insulin
•? of which existing meds to continue, generally just metformin
Sitagliptin
Goal Not
Met
Add Sulfonylurea
(consider TZD)