Type II Diabetes
advertisement

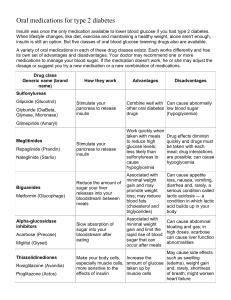
Type II Diabetes Heidi Chamberlain Shea, MD Endocrine Associates of Dallas Points of Discussion Type II Diabetes Background Diagnosis Pathophysiology Oral treatments Office visits Type II Diabetes Epidemic Parallels the rise in obesity Genetics Increase intake Decreased activity Obesity causes earlier disease High risk ethnic groups Indian, African American and Hispanic Obesity Trends* Among U.S. Adults (*BMI 30, or about 30 lbs overweight for 5’4” person) 1991 1996 2003 No Data <10% 10%–14% 15%–19% 20%–24% ≥25% Type II Diabetes Insulin resistance ß-Cell secretory dysfunction Relative insulin deficiency Onset in puberty Acanthosis Nigricans Female Diagnosis of Diabetes ADA Expert Committee Symptoms of diabetes plus a random plasma glucose concentration of 200 mg/dL, regardless of the time since last meal FASTING (8 hrs) NORMAL < 110 mg/dl (6.1 mM) IMPAIRED 110-125 mg/dl DIABETES >= 126 mg/dl (7 mM) ORAL GTT* < 140 mg/dl (7.8 mM) 140-199 mg/dl >=200 mg/dl (11.1 mM) * Not recommended for routine clinical use Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2002;25 Suppl 1 Metabolic Consequences in Type 2 DM Impaired insulin secretion Increased Hepatic Decreased Muscle Glucose uptake Glucose Production Insulin Resistance Etiology of Type 2 Diabetes Impaired Insulin Secretion and Insulin Resistance Genes and environment Impaired insulin secretion + Insulin resistance Impaired glucose tolerance Type 2 diabetes Low Insulin Secretion and Sensitivity Both Predict Progression from IGT to Type 2 Diabetes Cumulative diabetes incidence over 4 years 40 30 20 Low 10 High 0 Low High Early-phase insulin secretion IGT=impaired glucose tolerance Weyer C et al. Diabetes Care. 2001;24:89-94 Decline of -Cell Function in the UKPDS Illustrates Progressive Nature of Diabetes -cell function 100 (% of normal by HOMA) 80 Time of diagnosis ? 60 40 Pancreatic function = 50% of normal 20 0 10 9 8 7 6 5 4 3 2 1 0 HOMA=homeostasis model assessment Years Adapted from Holman RR. Diab Res Clin Pract. 1998;40(suppl):S21-S25; UKPDS. Diabetes. 1995;44:1249-1258 1 2 3 4 5 6 High FFA Levels Cause Peripheral and Hepatic Insulin Resistance Glucose Measurements During High Insulin Levels 500 Insulin Insulin + fat infusion 400 300 * 200 * 100 0 FFA=free fatty acid Peripheral glucose uptake Boden G, Chen X. J Clin Invest. 1995;96:1261-1268 Hepatic glucose output *P<0.05 Mechanism of Glucotoxicity and Lipotoxicity The Glucosamine Hypothesis Glucose FFA Glucose Other pathways FFA Increased glucosamine Impaired insulin secretion from -cell Other pathways Insulin resistance in muscle and fat FFA=free fatty acid Hawkins M et al. J Clin Invest. 1997;99:2173-2182; Rossetti L. Endocrinology. 2000;141:1922-1925 Etiology of Type 2 Diabetes Impaired Insulin Secretion and Insulin Resistance Genes and environment Impaired insulin secretion Insulin resistance Impaired glucose tolerance Type 2 diabetes Progressive hyperglycemia and high free fatty acids Better Insulin Secretion After Improved Metabolic Control Response to Oral Glucose Before and After 3 Therapies Diet n=17 Plasma insulin (U/mL) Sulfonylureas n=26 Insulin n=7 50 * * * * * 25 * * * * * * * * After * *P<0.05 * Before * 0 0 1 2 3 0 * 1 2 Hours Kosaka K et al. Diabetologia. 1980;18:23-28 3 0 1 2 3 Regulation of Fasting Glucose • Hepatic glucose production is a primary factor determining fasting plasma glucose • Fasting hepatic glucose production is regulated by – Fasting (basal) plasma insulin – Hepatic sensitivity to insulin – Fasting substrate availability • In type 2 diabetes – Basal insulin secretion is impaired – Hepatic sensitivity to insulin is decreased Hepatic Resistance to Insulin in Type 2 Diabetes Hepatic Glucose Output During Graded Insulin Infusion Glucose output (mg/m2/min) 100 • • 50 0 Normal Type 2 diabetes * • * • • • 0 *P<0.05 • •* 50 •* • 100 150 Calculated portal plasma insulin (U/mL) Groop LC et al. J Clin Invest. 1989;84:205-213 • • Impaired Basal Insulin Secretion in Type 2 Diabetes Insulin Secretion Rate During Graded Glucose Infusion 30 Secretion/BMI 25 pmol x m2/(min x kg) Normal IGT Type 2 diabetes 20 15 10 5 0 100 IGT=impaired glucose tolerance BMI=body mass index 150 200 Plasma glucose (mg/dL) Byrne MM et al. Am J Physiol. 1996;270(4 pt 1):E572-E579 250 300 Regulation of Postprandial Glucose • A meal contains 6 to 20 times the glucose content of the blood • Normally, postprandial hyperglycemia is regulated by – Clearance of ingested glucose by the liver – Suppression of hepatic glucose production – Peripheral clearance of glucose Impaired Regulation of Postprandial Glucose • In impaired glucose tolerance or diabetes, glucose regulation is impaired by – Delayed and reduced insulin secretion – Lack of suppression of glucagon – Hepatic and peripheral insulin resistance Abnormal Acute Insulin Response to Intravenous Glucose in Type 2 Diabetes Plasma insulin (U/mL) Type 2 Diabetes Normal 100 1 00 80 80 Glucose 60 60 40 40 20 20 0 0 – 30 0 30 Glucose – 30 Minutes Robertson RP, Porte D Jr. J Clin Invest. 1973;52:870-876 0 30 OGTT-Stimulated Insulin Release in IGT and Type 2 Diabetes Insulin release (IR/G [pM/mM]) 120 100 80 60 40 20 0 OGTT=oral glucose tolerance test NGT=normal glucose tolerance IGT=impaired glucose tolerance Kahn SE et al. Diabetes. 1998;47:640-645 NGT n=56 IGT Type 2 diabetes n=28 n=10 Patterns of Glucose, Insulin, and Glucagon After Oral Glucose in Type 2 Diabetes Postprandial hyperglycemia Type 2 Diabetes 300 Normal 200 100 –60 0 60 180 Delayed and reduced 240 120 0 240 300 60 Minutes 360 Insulin (pmol/L) 120 Glucagon (fmol/L) Glucose (mg/dL) 400 –60 0 60 120 180 240 Minutes Mitrakou A et al. Diabetes. 1990;39:1381-1390 300 High and not suppressed 45 30 –60 0 60 120 Minutes 180 240 300 Insulin Resistance in IGT and Type 2 Diabetes Glucose disposal rate (mg/m2/min) 360 270 180 90 0 Normal IGT=impaired glucose tolerance Kolterman OG et al. J Clin Invest. 1981;68:957-969 IGT Type 2 diabetes Peripheral Insulin Sensitivity Decreases With Intra-abdominal Fat Intraabdominal fat (cm3) 300 Females r = -0.44 P=0.0024 250 200 150 100 Males 50 0 Females 0 1 2 3 4 5 6 7 8 9 10 11 Insulin sensitivity (x 10-4 min-1 • U-1 • mL-1) Cefalu WT et al. Metabolism. 1995;44:954-959 Males r= -0.68 P=0.005 Abnormal Glucose and Insulin Profiles in Type 2 Diabetes Glucose (mg/dL) 400 Insulin 120 (U/mL) Type 2 on diet only Normal 100 300 80 200 60 40 100 20 0600 1000 1400 1800 2200 0200 0600 B D L 0600 1000 1400 1800 2200 0200 0600 L D B Time of day B=breakfast; L=lunch; D=dinner Polonsky KS et al. N Engl J Med. 1988;318:1231-1239 Summary of Pathophysiology • Type 1 diabetes – The main abnormality is insulin deficiency • Type 2 diabetes – Both insulin deficiency and insulin resistance contribute –Free Fatty Acids –Increased Glucagon • Glucotoxicity and lipotoxicity – Poor metabolic control worsens insulin deficiency and insulin resistance Summary of Pathophysiology Basal hyperglycemia Basal insulin levels Hepatic response determine fasting plasma glucose Postprandial hyperglycemia Early insulin release Glucagon suppression Hepatic and muscle responses to insulin determine postprandial glucose Pharmacologic Treatment Insulin Secretagogues Sulfonylureas Meglitanides Insulin Sensitizers Metformin Thiazolidinediones α-Glucosidase inhibitor Acarbose, miglitol Insulin Sulfonylureas Older Newer Glyburide Micronase, Diabeta Glyburide Glynase Glipizide Glucotrol Glucotrol XL Glimepiride Amaryl Chlorpropamide Diabenase Tolazamide Acetohexamide Dymelor Tolbutamide Insulin Secretion by the -Cell Roles of Glucose, K+, and Ca2+ Glucose entry Insulin secretion GLUT2 glucose transporter Insulin secretory granules Glucokinase Ca2+ Glucose metabolism K+ ADP/ATP Ca2+ K+ K+ ADP/ATP SUR 1 Kir 6.2 K+ Potassium (KATP) KATP channel subunits: channel SUR 1=regulatory subunit; Kir 6.2=inward rectifying channel closes Ca2+ Ca2+ Ca2+ Calcium channel opens Insulin Secretagogues Sulfonylureas, Repaglinide, and Nateglinide Mechanism of action Increase basal and/or postprandial insulin secretion Efficacy depends upon Functioning -cells Power Sulfonylureas, repaglinide: decrease A1C 1%–2% Nateglinide: decreases A1C 0.5%–1% Dosing Sulfonylureas: 1 or 2 times daily Repaglinide, nateglinide: 3 or 4 times daily with meals Side effects Weight gain, allergy (rare) Main risk Hypoglycemia Riddle MC. Am Fam Physician. 1999;60:2613-2620; Wolffenbuttel et al. Drugs. 1995;50:263-288; Horton ES et al. Diabetes Care. 2000;23:1660-1665; Hanefeld M et al. Diabetes Care. 2000;23:202-207; Medical Management of Type 2 Diabetes. 4th ed. Alexandria, Va: ADA; 1998 Biguanides Metformin Primary mechanism of action Decreases hepatic glucose production Efficacy depends upon Presence of insulin Power Dosing Decreases A1C 1%–2% 2 or 3 times daily (metformin) 1 or 2 times daily (metformin XR) Side effects Diarrhea, nausea Main risk Lactic acidosis Riddle MC. Am Fam Physician. 1999;60:2613-2620; Cusi K et al. Diabetes Rev. 1998;6:89-131 α-Glucosidase Inhibitors Acarbose and Miglitol Mechanism of action Delay carbohydrate absorption Efficacy depends upon Postprandial hyperglycemia Power Decrease A1C 0.5%–1% Dosing 3 times daily Side effects Flatulence Main risk Liver enzyme elevation (rare) Riddle MC. Am Fam Physician. 1999;60:2613-2620; Lebovitz HE. Endocrinol Metab Clin North Am. 1997;26:539-551 Glitazones (TZDs) Pioglitazone and Rosiglitazone Mechanism of action Enhance tissue response to Efficacy depends upon insulin Presence of insulin and resistance to its action Power Decrease A1C 0.9%–1.6% Dosing Once daily Side effects Edema, weight gain, anemia Main risk Congestive heart failure Riddle MC. Am Fam Physician. 1999;60:2613-2620; Zinman B. Diabetes Obesity Metab. 2001;3(suppl 1):S34-S43; Actos (pioglitazone hydrochloride) package insert; Avandia (rosiglitazone maleate) package insert Antihyperglycemic Agents Major Sites of Action -Glucosidase inhibitors Glitazones – Plasma glucose Carbohydrate absorption Glucose uptake GI tract Metformin + + Glucose production – Liver – Insulin secretion Secretagogues + Pancreas Muscle/Fat – Injected insulin + Glucose Profiles in Type 2 Diabetes Effects of Correcting Basal vs Postprandial Hyperglycemia Plasma glucose (mg/dL) 300 A1C 9% A1C 7% 200 A1C 6% 100 Normal—A1C 5% 0800 1200 1800 Time of day 0800 Effects of Metformin Added to a Sulfonylurea Glucose and Insulin Profiles Glipizide alone Glipizide + metformin Glucose Insulin pmol/L 500 mg/d 300 400 L 200 300 200 100 100 Meal Meal Meal Meal 0 0 0800 1200 1800 0800 2400 Time of day Jeppesen J et al. Diabetes Care. 1994;17:1093-1099 1200 1800 2400 Oral Antihyperglycemic Monotherapy Maximum Therapeutic Effect on A1C Nateglinide Acarbose Repaglinide Rosiglitazone Pioglitazone Glimepiride Glipizide GITS Metformin 0 -0.5 -1.0 -1.5 Reduction in A1C (%) Diabetes Care. 2000;23:202-207; Precose (acarbose) package insert; Drugs. 1995;50:263-288; J Clin Endocrinol Metab. 2001;86:280-288; Diabetes Care. 2000;23:1605-1611; Diabetes Care. 1996; 19:849-856; Diabetes Care. 1997;20:597-606; Am J Med. 1997;102:491-497 -2.0 Treatment of Postprandial Glycemia Conclusions From Studies Most oral agents control mainly fasting (basal) hyperglycemia Acarbose, miglitol, and nateglinide have the greatest effect on postprandial increments and the least ability to reduce A1C Time Course of Action of Oral Antihyperglycemics Effect on Fasting Plasma Glucose Rosiglitazone (8 mg) added to metformin FPG (mg/dL) Metformin (up to 2550 mg) Glimepiride (4 mg) 0 -20 -40 -60 0 2 4 8 12 Weeks of treatment DeFronzo RA et al. N Engl J Med. 1995;333:541-549; Fonseca V et al. JAMA. 2000;283:1695-1702; Goldberg RB et al. Diabetes Care. 1996;19:849-856 16 Selected Insulin Secretagogues Dosing Information Generic Name Effect Dose Strengths Recommended Usual Dose Range Maximal Glimepiride 1, 2, or 4 mg 1–8 mg 4 mg qd Glipizide GITS 2.5, 5, or 10 mg 2.5–20 mg 5 or 10 mg qd Glyburide 1.25, 2.5, or 5 mg 1.25–20 mg 5 or 10 mg bid Nateglinide 60 or 120 mg 180–360 mg 120 mg tid Repaglinide 0.5, 1, or 2 mg 1–16 mg 4 mg qid Metformin, -Glucosidase Inhibitors, Glitazones Dosing Information Generic Name Effect Dose Strengths Recommended Dose Range Usual Maximal Metformin 500, 850, or 1000 mg 500 mg qd to 850 mg tid 1000 mg bid Metformin XR 500 mg 500 mg qd to 1000 mg bid 1000 mg bid Acarbose 25, 50, or 100 mg 25–100 mg tid 50 mg tid Miglitol 25, 50, or 100 mg 25–100 mg tid 50 mg tid Pioglitazone 15, 30, or 45 mg 15–45 mg qd 45 mg qd Rosiglitazone 2, 4, or 8 mg 4–8 mg daily 4 mg bid Metabolic Effects of Oral Antihyperglycemics Sulfonylureas Meglitinides Acarbose Pioglitazone Miglitol Rosiglitazone Metformin Weight or LDL cholesterol or HDL cholesterol or Triglycerides or Medical Benefits Substantiated in Published Clinical Trials Microvascular Cardiovascular Insulin Yes Yes? Sulfonylureas Yes No Metformin Yes Yes? -Glucosidase inhibitors No No Glitazones (TZDs) No No Repaglinide, nateglinide* No No * Recently available agents with few trials documenting long-term outcomes Severe Adverse Effects Hypoglycemia Insulin Sulfonylureas Metformin -Glucosidase inhibitors Glitazones (TZDs) Repaglinide, nateglinide* Yes Yes No No No Yes CV No No No No Yes (CHF) No Lactic acidosis No No Yes No No No * Recently available agents with few trials documenting long-term outcomes CV=cardiovascular; CHF=congestive heart failure Efficacy of Oral Antihyperglycemics Declines With Time A1C rises at ~0.2% to 0.3% yearly on stable therapy This rate is the same as for diet alone, sulfonylureas, and metformin -Cell function declines at the same rate with all these treatments Combination treatments are routinely needed UKPDS Group. Diabetes. 1995;44:1249-1258; Turner RC et al. JAMA. 1999;281:2005-2012 Secondary Failure of Monotherapy Overweight Patients in the UKPDS Percent with A1C <7% on monotherapy Diet 3 years 23 6 years 12 9 years 11 Sulfonylureas 45 28 21 Metformin 44 34 13 Turner RC et al. UKPDS 49. JAMA. 1999;281:2005-2012 Insulin Therapy Who should be on insulin? Insulin Deficient DKA Weight loss Patient on maximal oral therapy A1c not at goal Insulin Therapy Basal insulin plus secretagogue Lantus HS (10 units) NPH HS (10 units) Bolus and Basal 75/25 Novolog or Humalog with NPH 70/30 Regular with NPH New Therapies Fat absorption blocker─orlistat (Xenical®) Amylin analogue─pramlintide (Symlin™) Oral intestinal lipase inhibitor Approved for obesity, proposed for diabetes Slows gastric emptying, suppresses glucagon, increases satiety Requires injection GLP-1 analogues/agonists─exendin-4 (ByettaTM) Potentiate insulin secretion, suppress glucagon May promote -cell neogenesis Require injection GLP-1=glucagon-like peptide Summary of Oral Antihyperglycemic Agents Four major classes of oral agents acting at different sites are available Fasting and preprandial glucose are reduced by sulfonylureas, repaglinide, metformin, and glitazones (TZDs), with lesser effects on postprandial increments Postprandial glucose increments are reduced best by -glucosidase inhibitors and nateglinide A1C reductions are similar using sulfonylureas, metformin, and glitazones Secondary failure to monotherapy and oral therapies routinely occurs Complications of Diabetes Type I and II Atherosclerosis Stroke Heart disease Retinopathy Leading cause of blindness Neuropathy Peripheral Autonomic Nephropathy Leading cause of end stage renal disease One out of every 3 patients on dialysis or transplant program Risk of Progression of Complications: DCCT Study 15 13 11 Relative Risk Diabetic retinopathy Nephropathy Neuropathy Microalbuminuria 9 7 5 3 1 7 8 9 10 HbA1c % 11 12 Skyler JF. Endocrinol Metab Clin North Am. 1996;25:243-254. Treatment of Diabetes Treatment goals Type I A1c < 7.0 Type II A1c < 6.0 Minimize hypoglycemia Maximize participation in usual activities Goals of Diabetes Treatment Early detection of complications: Annual retinal exam Annual renal exam (microalbuminuria, Creatinine) Frequent lower extremity examinations Diabetes Management Home blood glucose monitoring 4 times daily Periodically check at 3 AM Office visit Q3-4 months PM insulin Every year Monitor HgbA1c Adjustment of medication Examine injection sites TSH Lipid panel Hypertrophy Lipoatrophy Blood pressure Weight Conclusions Type II Diabetes is multifactorial Therapy Metabolic syndrome Fatty acid excess Multiple agents needed With time insulin is needed Tight control prevents complications Silent disease Assume 10 years of disease and vascular damage at time of diagnosis Conclusions Therapy Diet therapy Exercise Medical therapy Multiple agents needed With time insulin is needed Insulin Oral agents Adjunctive therapies Education Diabetes Team MD, RN CDE, Dietitian, Social work and Psychiatry Diet Therapy Lose weight Decrease portion size 1 pound per week Decreased intake of 500-1000 calories per day 3500-7000 calories per week Decrease carbohydrate intake Lower fat intake Increase activity Walk the dog you don’t have one) (even if Pramlintide Reduces A1C and Weight Combination With Insulin for Type 2 Diabetes Placebo + insulin Pramlintide 150 g tid + insulin A1C % kg A1C Placebo-subtracted difference 0.4% Weight 1.5 0 1.0 -0.2 0.5 *P<0.01 -0.4 * * -0.6 -0.8 * 0 13 26 0 -0.5 * *P<0.01 -1.0 * -1.0 * Placebo-subtracted difference 2.5 kg 39 -1.5 52 0 Week Ratner RE et al. Diabetes Technol Ther. 2002;4:51-61 * * 13 26 * 39 52 Synthetic Exendin-4 Reduces A1C and Weight 28-Day Treatment of Type 2 Diabetes 109 Patients With Continued Treatment on Sulfonylurea, Metformin, or Both Placebo AC2993 bid A1C A1C Placebo-subtracted difference -0.8% % 0 -0.2 -0.3% Placebo-subtracted difference -2.9 lb lb 3.0 2.0 -0.4 1.0 -0.6 P0.006 -0.8 -1.0 Weight -1.1% -1.2 0 -1.0 -2.0 Fineman MS et al. Diabetes 2002;51:A85 2.0 -0.9 Orlistat Reduces A1C and Weight Treatment of Patients With Type 2 Diabetes % body weight change 0 A1C (%) 9.0 8.5 -2 P=0.0003 8.0 P=0.006 -4 7.5 7.0 Placebo Orlistat 120 mg tid -4 0 12 24 -6 36 48 Week Hanefeld M et al. Diabetes Obes Metab. 2002;4:415-423 -4 0 12 24 36 48 Insulin Action in Muscle and Fat Cells Mobilization of GLUT4 to the Cell Surface Plasma membrane Insulin receptor Intracellular signaling cascades Intracellular GLUT4 vesicles Insulin GLUT4 vesicle mobilization to plasma membrane GLUT4 vesicle integration into plasma membrane Glucose entry into cell via GLUT4 GLUT4=glucose transporter 4