Quantum Slides
advertisement

Chemistry 111
Sections 10.1 – 10.4
Chemistry 111
1
Chapter 10
• Chapter 10 Topics
– Electromagnetic (Light)
Spectrum
– Spectroscope
– Bohr Model
– Quantum Model
(Schrödinger)
•
•
•
•
•
Principle
Orbital Shape
Exclusion
Electron Configuration
Noble Gas Cores
– Periodic Table Trends
– Valence Electrons
• What we’ll do:
– Light & Spectrometer
Demonstration
– Bohr Model Animation
– Quantum Model –
Beginning
– Computerized Orbitals
(breath)
– Quantum Model Extension
– Periodic Trends
– Valence Electrons (ns2np5)
2
Electromagnetic Spectrum (Light)
• Visible Light (400 – 700 nm)
Violet
400 nm
Green
575 nm
Blue
491 nm
Orange
647 nm
Yellow
585 nm
Red
700 nm
3
Electromagnetic Spectrum (Light)
4
Spectroscope / Spectrometer
5
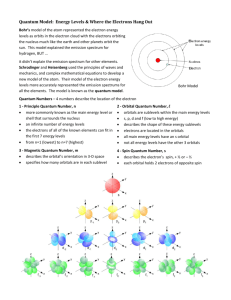
Bohr Model of the Atom
• View Animations Online
• View UV/Visible Spectrum of:
–
–
–
–
–
Hydrogen Lamp
Neon Lamp
Mercury Lamps (overhead lights)
Projector Lamp (Incandescent / W)
Fire works.
6
Quantum Model
• Bohr Model works perfectly for Hydrogen
• Doesn’t work well for rest of the periodic
table.
• Refined with the Schrodinger Equation
–
–
–
–
Still have Bohr Energy Levels
Add “sublevels” – shape of the orbital
Multiple orbitals per sublevel.
2 Electrons per orbital
7
Quantum Model - n
• Principle Quantum Number (n)
– n can be {1, 2, 3, 4, 5, 6, 7}
– Corresponds to the ROWS on the periodic
table
8
Quantum Model - Sublevels
• Sublevels = Shape
• Show Web Site with shapes.
http://www.chem.purdue.edu/gchelp/aos/1s.html
• Possible Sublevels:
– s, p, d, f
– # of possible sublevels is same as value of n:
•
•
•
•
•
n = 1:
s sublevel only
n = 2:
s&p
n = 3:
s, p & d
n = 4:
s, p, d & f
We never need more than f – there aren’t enough stable
elements.
9
Quantum Model – the One s Orbitals
• “s” sublevels are “spherical” (round)
• These are the Alkali Metal & Alkaline
Earth Metals!
10
Quantum Model – the Three p Orbitals
• “p” orbitals are sketched as pear-shapes (middle).
• There is one along each axis – x, y, z for a total of 3.
• These are the {Al, C, N, O} families, halogens, noble gases.
Left: true shape
Middle: sketch
Right: All at once.
11
Quantum Model – the Five d Orbitals
• 4 of the “d” are 4-petal flowers. The 5th is weird! These are the
transition elements!
• These are the
transition metals.
B: True Shape
C & D: Sketch
E: The funny one.
12
Quantum Model – the Seven f Orbitals
These are the Lanthanides & Actinides
13
Summary
# of
Orbitals
# of
Electrons
Total
Electrons
1
2
2
8
n
1
Sublevels
s only
2
s
1
2
p
3
32=6
s
1
2
p
3
6
d
5
52=10
s,p,d,f
1, 3, 5, 7
3
4
18
32
14
Sublevels & the Periodic Table
15
Team Exercise
• This chapter is mainly qualitative
– We will use the team activity to actively review the
topics.
– Discuss what you don’t fully understand.
– Note topics that you want me to go over.
• Review your chapter notes.
– Update them with good ideas from teammates.
• GOAL / ASSIGNMENT: Spend time actively
reviewing the material.
• Complete your active learning log.
16
Orbitals / Energy Levels
• Bigger orbitals have higher energy, electrons
want to fill in the bottom levels first.
• Filling Order:
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f 5g
6s 6p 6d 6f 6h 6i
7s 7p 7d 7f 7h 7i 7j
1s
2s
3s
4s
5s
6s
7s
2p
3p
4p
5p
6p
7p
3d
4d
5d
6d
7d
4f
5f 5g
6f 6h 6i
7f 7h 7i 7j
17
Electron Configuration
• On Chalkboard:
– Energy Level Diagrams
– Shorthand #1: 1s22s23p6…
– Shorthand #2: Noble Gas Core
18
Periodic Trends – Atomic Radii
19
Periodic Trends – Ionization Energy
20
Team Exercise Time
• This chapter is mainly qualitative
– We will use the team activity to actively review the
topics.
– Discuss what you don’t fully understand.
– Note topics that you want me to go over.
• Review your cheatsheets
– Update them with good ideas from teammates.
• GOAL / ASSIGNMENT: Spend time actively
reviewing the material.
• Complete your active learning log.
21