Diabetes Care
advertisement

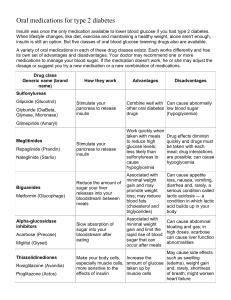
Diabetes Mellitus
Oral Hypoglycemic Agents
Dr. A. ghanei
endocrinologist
Non-Insulin Hypoglycemic
Agents
Oral
Biguanides
Sulfonylureas
Meglitinides
Thiazolidinediones
Alpha Glucosidase
inhibitors
Incretin Enhancers (DPPIV inhibitors)
Resin binder
Parenteral
Amylin analogs
Incretin mimetics
Sulfonylureas : stimulate β cells to produce
more insulin
Rel. Potency
bind to protein
1st generation
–
–
(1)Orinase
(tolbutamide)
(3)Tolinase (tolazamide)
(6)Diabinese (chlorpropamide)
2-(p-aminobenzenesulfonamido)-5-isopropyl -thiadiazole (IPTD)
was used in treatment of typhoid fever in 1940’s hypoglycemia
Currently > 12,000
–
may become dislodged delayed activity
2nd generation
–
–
–
–
(75)Glucotrol
(glipizide)
(150)Glucotrol XL (ex. rel. glipizide)
(150)Micronase, Diabeta (glyburide)
(250)Glynase (micronized glyburide)
3rd generation
–
(350)Amaryl
(glimepiride)
*Hydroxylation of the aromatic ring appears to be the most favored metabolic pathway
*Hydroxylated derivatives have much lower hypoglycemic activity
Glimepiride
(Amaryl)
1, 2, 4 mg
tablets
Glipizide
(Glucotrol,
Glucotrol XL)
(2.5), 5, 10 mg (XL)
tablets
Glyburide
(DiaBeta)
1.25, 2.5, 5 mg
tablets
Mechanism of action:
– presence of functional pancreatic beta cells is must
– SUs imitate the effects of glucose on beta cells
– SUs bind to SURs at the beta-cell potassium channels >
this closes the channels and blocks potassium ions from
leaving the cell > The membrane then becomes
depolarized and calcium channels open > Calcium ions
enter the cell and trigger the release of insulin > The
increased insulin concentration lowers blood glucose
levels
Mechanism of action
Pharmacology - Sulfonylureas
Compound
•
•
•
•
Mechanism
Closes KATP channels on β-cell plasma membranes
Action(s)
Insulin secretion
Advantages
• Generally well tolerated
• Reduction in cardiovascular events and mortality (UKPDS
f/u)
Disadvantages
• Relatively glucose-independent stimulation of insulin
secretion: Hypoglycemia, including episodes necessitating
hospital admission and causing death
• Weight gain
• Primary and secondary failure
Glibenclamide/Glyburide
Glipizide
Gliclazide
Glimepiride
Biguanides : improves insulin’s ability to
move glucose into cells (esp. muscle)
R
R
N
R
N
R
N
R
N
N
H
Metformin
N
R
N
R
H
- Glucophage®, Fortamet®,
Riomet®
N
N
H
H
N
H
+
HCl
- mechanism improves insulin sensitivity by increasing peripheral
glucose uptake and utilization.
- Zhou et al (2001) showed that metformin stimulates the hepatic
enzyme AMP-activated protein kinase
- Metformin was first described in the scientific literature in 1957 (Unger
et al).
- It was first marketed in France in 1979 but did not receive FDA approval
for Type 2 diabetes until 1994.
Metformin is a widely used monotherapy, and also used in
combination with the sulfonylureas in treatment of type 2
diabetes
*only anti-diabetic drug that has been proven to reduce the complications of diabetes, as evidenced in a large study of overweight patients with
diabetes (UKPDS 1998).
Biguanides
Metformin is a biguanide that works
mainly by:
Suppressing excessive hepatic glucose
production
Increasing glucose utilization in peripheral
tissues to a lesser degree
It may also improve glucose levels by
reducing intestinal glucose absorption
Metformin
Glucophage
500, 850, 1000 mg
tablets
(Glucophage XR)
500, 750 mg XR
tablets
Pharmacology - Biguanides
Mechanism
Activates AMP-kinase
Action(s)
• Hepatic glucose production
• Intestinal glucose absorption
• Insulin action
Glucose
Lowering
Effect
• Fasting
• Post Prandial
Advantages
• No weight gain
• No hypoglycemia
• Reduction in cardiovascular events and mortality
(UKPDS f/u)
Disadvantages • Gastrointestinal side effects (diarrhea, abdominal
cramping)
• Lactic acidosis (rare)
• Vitamin B12 deficiency
• Contraindications: reduced kidney function
Thiazolidinediones (TZD’s) : make
cells more sensitive to insulin (esp. fatty cells)
O
Pioglitazone
N
O
S
NH
- Actos®, Avandia®
O
5-{4-[2-(5-Ethyl-pyridin-2-yl)-ethoxy]-benzyl}-thiazolidine-2,4-dione
- binds to and activates (PPARγ).
- PPARγ is a member of the steroid hormone nuclear receptor
superfamily, and is found in adipose tissue, cardiac and skeletal muscle,
liver and placenta
- upon
activation of this nuclear receptor by a ligand such as a TZD,
PPARγ–ligand complex binds to a specific region of DNA and thereby
regulates the transcription of many genes involved in glucose and fatty
acid metabolism.
PPAR - γ
- Marketed in USA in August of 1999
Pioglitazone
(Actos)
15, 30, 45 mg
tablets
Rosiglitazone
(Avandia)
2, 4, 8 mg
tablets
Pharmacology – Thiazolidinediones
(TZD)
Compound
Rosiglitazone (Avandia®)
Mechanism
Activates the nuclear transcription factor PPAR-
Action(s)
Peripheral insulin sensitivity
Advantages
No hypoglycemia
Disadvantages
•
•
•
•
•
•
•
LDL cholesterol
Weight gain
Edema
Heart failure (CI with stages III and IV)
Bone fractures
Increased cardiovascular events (mixed evidence)
FDA warnings on cardiovascular safety
ADA. V. Diabetes Care. Diabetes Care 2012;35(suppl 1):S22.
Adapted with permission from Silvio Inzucchi, Yale University.
Αlpha – glycosidase inhibitors :
Block enzymes that help digest starches slowing
the rise in BS.
AGI’s
- Precose ® (acarbose),
- Glyset ® (miglitol)
H
O
H
O
H
O
N
H
O
O
H
1-(2-Hydroxy-ethyl)-2-hydroxymethylpiperidine-3,4,5-triol
Pharmacology – Alpha-Glucosidase
Inhibiters
Compound
• Acarbose
• Miglitol
Mechanism
Inhibits intestinal α-glucosidase
Action(s)
Intestinal carbohydrate digestion and absorption
slowed
Advantages
• Nonsystemic medication
• Postprandial glucose
Disadvantages
• Gastrointestinal side effects (gas, flatulence,
diarrhea)
• Dosing frequency
ADA. V. Diabetes Care. Diabetes Care 2012;35(suppl 1):S22.
Adapted with permission from Silvio Inzucchi, Yale University.
Meglitinides : Stimulate more insulin
production ; dependant upon level of glucose present
Meglitinides
O
- Prandin ® (repaglinide)
N
O
OH
NH
O
2-Ethoxy-4-{[3-methyl-1-(2-piperidin-1-yl-phenyl)-butylcarbamoyl]-methyl}-benzoic acid
- Starlix ® (nateglinide)
NH
O
O OH
2-[(4-Isopropyl-cyclohexanecarbonyl)-amino]-3-phenyl-propionic acid
Meglitinides
Examples: repaglinide, nataglinide
It is a benzoic acid derivative and a short-acting
insulin releaser.
It stimulates the release of insulin from the
pancreatic beta cells by closing ATP-sensitive
potassium channels.
It has no significant effect on plasma lipid levels
Rapid onset and short duration of action make
multiple daily doses necessary (take it
immediately before each meal!).
Pharmacology – Meglitinides
Compound
• Repaglinide (Prandin®)
• Nateglinide (Starlix®)
Mechanism
Closes KATP channels on β-cell plasma membranes
Action(s)
Insulin secretion
Advantages
Accentuated effects around meal ingestion
Disadvantages
• Hypoglycemia, weight gain
• Dosing frequency
ADA. V. Diabetes Care. Diabetes Care 2012;35(suppl 1):S22.
Adapted with permission from Silvio Inzucchi, Yale University.
Diabetes – Oral Medications
Summary
5 Classes :
Sulfonylureas
Biguanides
stimulate β cells
improves insulin’s ability to move glucose
Thiazolidinediones cells more sensitive to insulin
Alpha-glycosidase inhibitors
Block enzymes that help
digest starches
Meglitinides
stimulate β cells (dependant upon glucose conc.)
Pharmacology – Bile Acid
Sequestrants
Compound
Colesevelam (Welchol®)
Mechanism
Binds bile acids/cholesterol
Action(s)
Bile acids stimulate receptor on liver to produce
glucose
Results
• Lowers fasting and post prandial glucose
Advantages
• No hypoglycemia
• LDL cholesterol
Disadvantages
• Constipation
• Triglycerides
• May interfere with absorption of other
medications
ADA. V. Diabetes Care. Diabetes Care 2012;35(suppl 1):S23.
Adapted with permission from Silvio Inzucchi, Yale University.
Drug Pearls
Medication
PRO
CON
Metformin
Low cost, A1c lowering, +
CV effects, weight loss,
PCOS
Renal or hepatic impairment
Sulfonylurea
Low cost, A1c lowering
Hypoglycemia, treatment
failure
Meglitinides
Erratic meals, renal
insufficiency
Hypoglycemia, treatment
failure
Pioglitazone
Insulin resistance, decrease
in adipose tissue, TG
reduction
Edema, wt gain, CI with HF
class III and IV
α-glucosidase inhibitors
Patients with constipation
Long duration of T2DM,
patients with GI problems
DPP-4
Well tolerated
? long term safety
Typical A1c Reductions
Monotherapy
Route of Administration
A1c (%) Reduction
Sulfonylurea
PO
1.5-2.0
Metformin
PO
1.5
Glitazones
PO
1.0-1.5
Meglitinides
PO
0.5-2.0
α-glucosidase inhibitors
PO
0.5-1.0
DPP-4
PO
0.5-0.7
Insulin
Injectable
Open to target
Unger J et al. Postgrad Med 2010; 122: 145-57
Fasting vs. Postprandial Effect
Mostly targets FASTING
hyperglycemia
Mostly targets POSPRANDIAL
hyperglycemia
Insulin (long and intermediate
action)
Insulin (regular, rapid-action)
Colesevelam
α-glucosidase inhibitors
Metformin
Meglitinides
Pramlinitide
DPP-4 inhibitors
GLP-1 agonist
PHARMACOLOGIC MANAGEMENT
The stepwise approach described in the 1998 CDA
Clinical Practice Guidelines implied that it was
acceptable to wait for up to 8 to 16 months before
implementing aggressive therapy to treat hyperglycemia.
It is now recommended that the management
regimens of patients with type 2 diabetes be
tailored to the individual patient, aiming for
glycemic targets as close to normal as possible
and, in most people, as early as possible.
Multiple therapies may be required to achieve
optimal glycemic control in type 2 diabetes.
The choice of antihyperglycemic agent(s) should
be based on the individual patient.
Target A1C should be attainable within 6 to 12
months.
Type 2 Diabetes Recommendations
Metformin + lifestyle changes at diagnosis providing no
contraindication
– Medications are ALWAYS to be used in combination with
healthy meal planning and regular physical activity (150
minutes per week)
If marked elevation of A1c /blood glucose and/or symptomatic
consider insulin (+ or – other agents) from the onset
If noninsulin monotherapy at maximal tolerated dose does not
achieve /maintain the A1c goal over 3–6 months, add a second
oral agent, a GLP-1 receptor agonist, or insulin
ADA. V. Diabetes Care. Diabetes Care 2012;35(suppl
1):S21
Considerations When Selecting Therapy
How long has the patient had diabetes (duration of
disease – preservation of β-cell function)?
Which blood glucose level is not at target (fasting,
postprandial, or both)?
The degree of A1c lowering effect required to achieve
goal?
Side effect profile and the patients tolerability?
Co – existing conditions ( CVD, osteoporosis, obesity,
etc)?
Patient preference for route of administration (oral,
injection)?