Chapter 26: Energy in the Atmosphere
advertisement

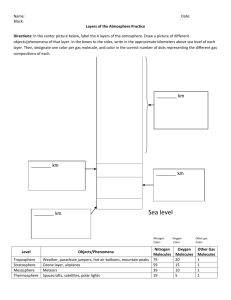
Integrated Science Unit 9, Chapter 26 Unit Nine: Energy in the Earth System Chapter 26 Energy in the Atmosphere 26.1 The Atmosphere 26.2 Layers of the Atmosphere 26.3 Energy in the Atmosphere Chapter 26 Learning Goals Learn about the thermal structure and chemical composition of Earth’s atmosphere. Compare and contrast the atmospheres of Venus, Earth, and Mars. Find out how life on Earth has changed Earth’s atmosphere. Build and calibrate a barometer to measure atmospheric pressure. Learn how changing atmospheric pressure affects the weather. Describe the layers and the corresponding temperature changes in the atmosphere. Learn about ozone’s helpful role in the stratosphere and harmful role in the troposphere. Measure ozone levels in your school. Describe energy transfer in the atmosphere. Discuss greenhouse gas emission reduction and other means of slowing global warming. Model factors affecting Earth’s temperature, including greenhouse gases, thermal buffering properties of ice, and water’s high specific heat. Chapter 26 Vocabulary Terms atmosphere atmospheric ionosphere pressure mesosphere barometer ozone chlorofluorocarbons stratosphere exosphere thermosphere greenhouse global effect warming troposphere 26.1 The Atmosphere Earth’s atmosphere is a layer of gases surrounding the planet, protecting and sustaining life. — — — It insulates us so that we don’t freeze at night. Its ozone layer protects us from the sun’s ultraviolet rays. It also contains the carbon dioxide needed by plants for photosynthesis, and the oxygen we need to breathe. 26.1 What's in Earth's atmosphere? This wonderful protective layer exists around Earth because our planet has just the right balance of size and distance from the sun. Nitrogen gas makes up about 78 percent of Earth’s atmosphere. The second most abundant gas is oxygen, which makes up 21 percent of Earth’s atmosphere. 26.1 Nitrogen Cycle Nitrogen is released into the air by volcanoes and decaying organisms. Nitrogen is not absorbed directly from the air. Instead, the nitrogen is changed into nitrates (NO3) by nitrogen-fixing organisms in the soil. Plants absorb nitrates from the soil and use them to make proteins. We eat plants (especially their seeds) or meat to obtain these proteins. 26.1 Why is Earth different? The atmospheres of Venus, Earth, and Mars were formed in similar ways, so we might expect them to contain similar elements. Through photosynthesis, life on Earth has actually changed the planet’s atmosphere. 26.1 Atmospheric pressure Atmospheric pressure is a measurement of the force of air molecules in the atmosphere at a given altitude. The pressure of the atmosphere changes as you rise above sea level. 26.1 Atmospheric pressure At sea level, the weight of the column of air above a person is about 9,800 newtons (2,200 pounds)! This is equal to the weight of a small car. Why aren’t we crushed by this pressure? 26.1 Measuring Pressure 26.1 Pressure changes with altitude 26.1 The Atmosphere Key Question: Can you measure atmospheric pressure? *Read text section 26.1 BEFORE Investigation 26.1 Parts of an Aneroid Barometer 26.2 Layers of the Atmosphere Four — — — — Layers Thermosphere Mesosphere Stratosphere Troposphere* 26.2 Layers of the Atmosphere The atmosphere is divided into layers based on temperature changes. We live in the troposphere which is 0 to 11 kilometers above Earth's surface. Layers of the Atmosphere The four layers of the atmosphere include: 1. the troposphere, where we live; 2. the stratosphere, which contains the ozone layer; 3. the mesosphere, where meteors burn; 4. and the thermosphere, where satellites orbit Earth. 26.2 Layers of the Atmosphere Key Question: How much ozone is in our living areas? *Read text section 26.2 BEFORE Investigation 26.2 26.3 Energy in the Atmosphere Our sun converts 5 million tons of its own mass into energy every second. This process involves nuclear fusion. Even though Earth intercepts only a tiny fraction of the radiation broadcast by the sun into space, this radiation provides most of Earth’s thermal energy. 26.3 Greenhouse effect Imagine an empty bucket with a hole near the bottom. If you pour water into that bucket at the same rate that the water spills out the hole, the bucket will never get full. If you fill the bucket partially with pebbles, it will take longer for the water to get through the bucket and out the hole. 26.3 Greenhouse effect There are molecules in the atmosphere that act like the pebbles in the bucket. They make it take longer for the infrared radiation to escape back into space. 26.3 Greenhouse effect Adding greenhouse gases to the atmosphere is like adding more pebbles to the bucket. It takes longer for radiation to escape from the atmosphere, so Earth’s average temperature rises. Gulf Stream and global warming 26.3 Energy in the Atmosphere Key Question: What factors affect Earth's temperature? *Read text section 26.3 BEFORE Investigation 26.3