CH 6: Pathways that Harvest and Store Chemical
Energy
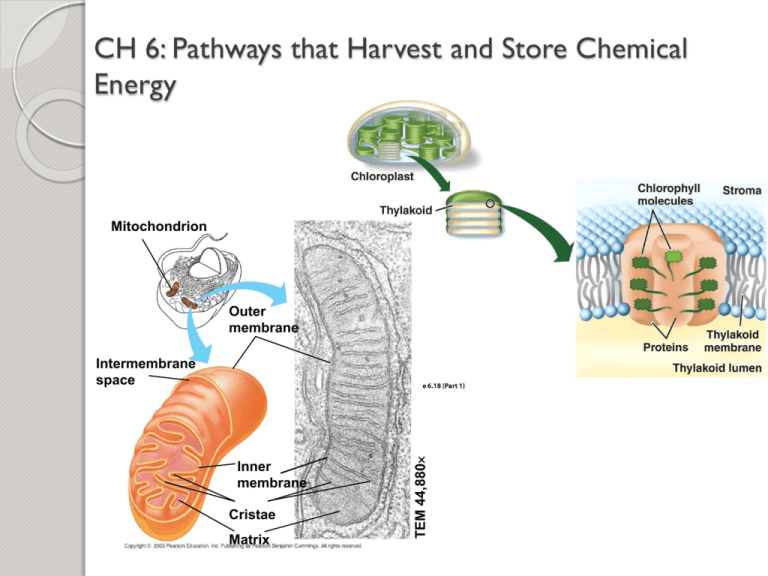
Mitochondrion
Outer
membrane
Inner
membrane
Cristae
Matrix
TEM 44,880
Intermembrane
space
For HW, read section 6.1 and define the following
vocabulary terms:
1.
2.
3.
4.
5.
6.
Reduction
Oxidation
Substrate-level phosphorylation
Oxidative phosphorylation
Chemiosmosis
ATP synthase
(2/4) BR: Energy review
1.
2.
3.
Describe the difference between endergonic and
exergonic reactions.
Describe the difference between anabolic and catabolic
reactions.
What is the energy molecule that cells use to do work?
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
Chemical energy available to do work is termed free
energy (G)
Five principles governing metabolic pathways:
1. Chemical transformations occur in a series of intermediate
reactions – a metabolic pathway or cascade
2. Each reaction is catalyzed by a specific enzyme
3. Most metabolic pathways are similar in all organisms
4. In eukaryotes, many metabolic pathways occur inside specific
organelles
5. Each metabolic pathway is controlled by enzymes that can
be inhibited or activated
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
Energy coupling: an energy-releasing (exergonic) reaction
provides energy for an energy-storing (endergonic) reaction
ATP (adenosine triphosphate)
◦ Energy released by exergonic reactions is stored in the
bonds of ATP
◦ When ATP is hydrolyzed, free energy is released to drive
endergonic reactions
ATP H2O ADP Pi freeenergy
(Pi refers to an inorganic phosphate molecule)
Energy Coupling – energy released from exergonic
reactions is used to drive endergonic reactions
Formation of ATP
requires energy
(endergonic)
Hydrolysis of ATP
releases energy
(exergonic)
ATP (adenosine triphosphate)
Which bond contains more free energy (G), the bond between
phosphate groups, or the O—H bond that forms after
hydrolysis of ATP?
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
Redox (oxidation–reduction) reactions transfer energy
◦ Reduction is the GAIN of one or more electrons
◦ Oxidation is the LOSS of one or more electrons
(“OIL RIG”…oxidation involves loss, reduction involves gain!)
Oxygen is one of the strongest oxidizing agents due to its
electronegativity! It loves to TAKE electrons from other
molecules, thus oxidizing them.
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
◦ Transfer of hydrogen atoms involve transfer of electrons
(H = H+ + e–)
◦ Energy in the reducing agent is transferred to the reduced
product
When the reducing
agent (compound A)
gives up electrons, it
is oxidized
When the oxidizing
agent (compound B)
takes electrons, it is
reduced
Oxidation, Reduction, and Energy
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
◦ Coenzyme NAD+ is a key electron carrier in redox
reactions (other examples are FAD & NADP+)
Reduction of NAD+ forms NADH
Formation of NADH is highly endergonic
NAD H 2e NADH
◦ In catabolic processes, oxidation releases energy that is
trapped by the reduction of coenzymes such as NADH
◦ Energy for anabolic processes is supplied by ATP
NAD+
NADH
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
Oxidative phosphorylation transfers energy from
NADH to ATP
NADH NAD H 2e energy
energy ADP Pi ATP
◦ Achieved via chemiosmosis—the diffusion of H+
(protons) across a membrane to drive the synthesis of
ATP
H+ diffuse through membrane protein ATP synthase
*Based on your vocab and your notes, draw a diagram of what
occurs during chemiosmosis
How do H+ diffuse through a membrane?
Chemiosmosis through ATP Synthase
Energy from
electron carriers
is used to actively
transport H+
against its
concentration
gradient
Diffusion of H+
through ATP
synthase provides
energy for ATP
formation
(2/4) Exit slip
Describe the energy transfer that takes place when ATP
is formed versus when it is hydrolyzed. When is energy
stored? When is it released?
If you can, draw a diagram showing ATP’s role in energy
coupling.
(1/30) BR: Vocabulary matching
the diffusion of protons (H+) across a membrane to
drive the synthesis of ATP
2. formation of ATP involving direct transfer of phosphate
to ADP
3. the gaining of one or more electrons
4. the loss of one or more electrons
5. energy requiring/absorbing
6. energy-releasing
7. membrane protein through which H+ diffuse to form
ATP
8. formation of ATP involving transfer of energy from
NADH to ADP
1.
(2/8) Bellringer: ATP & respiration
What does the word respiration mean?
2. How do photosynthesis and respiration cycle carbon
through the biosphere? Refer to the carbon-containing
molecules in each process.
3. Copy the diagram of ATP below (just the ATP) and
point out where in the molecule the energy is stored.
4. Describe the energy transfer that takes place between
the coenzymes ATP & NADH during oxidative
phosphorylation (check your notes).
Adenosine
Triphosphate
Adenosine diphosphate
Exit review
1.
Phosphate
P
Adenine
P
P
H2O
Hydrolysis
P
P
ADP + Pi
Ribose
ATP
Pi
Energy
BR: Energy review
Energy flows through the biosphere
◦ Enters as light and exits as heat
Chemicals (matter) are recycled through the biosphere
2. Photosynthesis uses CO2 to build organic molecules like
glucose (C6H12O6). Respiration breaks down organic
molecules and returns CO2 to the atmosphere.
6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play
Important Roles in Biological Energy Metabolism
Cellular respiration is a major catabolic pathway in which
glucose is oxidized and energy is released:
carbohydra te 6O2 6CO2 6H 2O chemical energy
Photosynthesis is a major anabolic pathway in which light
energy is converted to and stored as chemical energy
(glucose):
6CO2 6H 2O light energy 6O2 carbohydra te
6.2 Carbohydrate Catabolism in the Presence of Oxygen
Releases a Large Amount of Energy
Cellular Respiration – release of energy through the
oxidation of glucose (or other organic molecules)
◦ Aerobic respiration occurs in the presence of oxygen
I. Glycolysis
II. Pyruvate Oxidation
III. Citric Acid (Krebs) cycle
IV. Electron Transport Chain
Cellular Respiration: a series of small steps
Why doesn’t the oxidation of glucose occur in one single step?
Why doesn’t the oxidation of glucose occur in one
single step?
◦ It would be too explosive and not enough energy would
be harnessed
◦ There are other oxidizing agents for respiration in the
electron transport chain besides oxygen (the oxidizing
agent is the recipient of the electrons and therefore is
responsible for the oxidation of another molecule)
6.2 Carbohydrate Catabolism in the Presence of Oxygen
Releases a Large Amount of Energy
Glycolysis – conversion of 6-C glucose into two 3-C
pyruvate molecules
◦ Occurs in cytosol
◦ Products: 2 pyruvate, 2 ATP, 2 NADH
ATP is produced via substrate-level phosphorylation
Glycolysis converts
glucose into 2 molecules
of pyruvate
• Requires an “investment”
of 2 ATP at beginning
…phosphorylated glucose
is more reactive AND
cannot exit the cell
Glycolysis converts
glucose into 2 molecules
of pyruvate
Glycolysis converts
glucose into 2 molecules
of pyruvate
• Exergonic reactions at
the end of glycolysis
produce 2 NADH
and 4 ATP. ATP are
produced via
substrate-level
phosphorylation.
Oxidative phosphorylation
via chemiosmosis
Substrate level
phosphorylation
6.2 Carbohydrate Catabolism in the Presence of Oxygen
Releases a Large Amount of Energy
Pyruvate Oxidation: links glycolysis & citric acid cycle
◦ Occurs in mitochondrial matrix (2x per glucose)
◦ Products (total): 2 NADH, 2 CO2, & 2 acetyl CoA
pyruvate is oxidized to acetate which is then bound to
Coenzyme A (CoA), forming acetyl CoA
Mitochondrion
Outer
membrane
Intermembrane
space
Cristae
Matrix
TEM 44,880
Inner
membrane
Formation of Acetyl CoA
Citric Acid (Krebs)
Cycle
◦ Occurs in
mitochondrial
matrix (2x per
glucose)
◦ Products: 4 CO2,
6 NADH, 2 FADH2,
& 2 ATP
ATP is formed
from GTP via
substrate-level
phosphorylation
2-C acetyl group
gets oxidized, then
joins the 4-C
oxaloacetate to
form 6-C citrate
(citric acid)
GTP can transfer its
high-energy
phosphate to form
ATP
6.2 Carbohydrate Catabolism in the Presence of Oxygen
Releases a Large Amount of Energy
Electron Transport Chain: NADH & FADH2 are
oxidized to NAD+ & FAD, and O2 is reduced to form H2O
◦ Series of redox carrier proteins (the respiratory chain)
embedded in the inner membrane of mitochondria
Transport the electrons donated by NADH and FADH2
from one carrier to the next
Actively transport H+ into intermembrane space, setting
up a proton gradient
◦ Proton motive force: H+ diffuse through ATP synthase
to synthesize ATP by chemiosmosis
6.2 Carbohydrate Catabolism in the Presence of Oxygen
Releases a Large Amount of Energy
◦ Oxygen is the final electron acceptor at the end of the
ETC
◦ Products: 6 H2O, 32 ATP
ATP is produced via oxidative phosphorylation
◦ Complete chemical equation for aerobic respiration:
C6H12O6 + 6O2 6CO2 + 6H2O + Energy (36 ATP)
Electron Transport Chain & Chemiosmosis
ETC and chemiosmosis animation
Intermembrane
space
Proteins pass electrons from
NADH & FADH2. The energy
from the electrons is used to
pump H+ out of the matrix
across the inner membrane
of the mitochondrion.
O2 is the final
electron
acceptor at the
end of the ETC
Diffusion of H+ thru ATP
synthase produces ATP via
oxidative phosphorylation
Aerobic Respiration animation recap
Electrons
carried via
NADH
PYRUVATE
OXIDATION
(2/9) Group BR
Aerobic Respiration:
C6H12O6 + 6O2 6CO2 + 6H2O + Energy (36 ATP)
Cellular respiration involves the relocation of electrons,
which releases stored energy in organic molecules like
glucose. This energy is used to synthesize ATP. In the process,
glucose is oxidized and oxygen is reduced.
1. What molecules does glucose ultimately become once it
is fully oxidized during aerobic respiration?
2. What does oxygen become when it is reduced at the
end of the ETC?
3. The ATP from aerobic respiration represents ~40% of
the energy that was originally stored in glucose. What do
you think happens to the other 60%?
◦ Newborns contain a protein called thermogenin that
disrupts the H+ gradient in brown fat, preventing ATP
formation and increasing the release of heat.
4. Dinitrophenol (DNP) is an “uncoupler,” which means it interferes with the
flow of electrons during electron transfer and allows H+ to leak back into
the matrix across the inner membrane (see figure below). Fifty years ago,
DNP was given as a drug to help patients lose weight.
a. Why would taking DNP make someone lose weight?
b. Why would taking DNP be dangerous?
Intermembrane
space
Inner
membrane
Mitochondrial Matrix
6.3 Carbohydrate Catabolism in the Absence of Oxygen
Releases a Small Amount of Energy
Anaerobic respiration occurs entirely in the cytosol in the
absence of O2
I. Glycolysis (2 ATP)
II. Fermentation (0 ATP)
Fermentation regenerates NAD+ so glycolysis can continue
◦ Lactic Acid Fermentation – NADH reduces pyruvate
to lactic acid
Performed by animals, plants, microorganisms,…
Buildup of lactic acid in muscles inhibits muscle
contraction
◦ Alcoholic Fermentation – pyruvate is converted to
ethanol and CO2
Performed by yeast
Alcoholic and lactic acid fermentation
* In both cases NAD+ is regenerated from NADH
Stored ATP and
creatine
phosphate (CP)
can provide 10-15
seconds worth of
energy to muscles.
Anaerobic
respiration
provides energy
for a limited time
before lactic acid
buildup begins to
inhibit muscle
contractions.
6.4 Catabolic and Anabolic Pathways Are Integrated
Carbon skeletons (molecules with covalently linked carbon
atoms) can enter catabolic or anabolic pathways
Catabolism
◦ Polysaccharides are hydrolyzed to glucose, which enter
glycolysis
◦ Triglycerides (lipids) break down to fatty acids and
glycerol.
Fatty acids can be converted to acetyl CoA & enter the
Krebs cycle
◦ Proteins are hydrolyzed to amino acids that can feed into
glycolysis or the Krebs cycle
6.4 Catabolic and Anabolic Pathways Are Integrated
Anabolism
◦ Gluconeogenesis—Krebs cycle and glycolysis
intermediates can be reduced to form glucose
◦ Acetyl CoA can be used to form fatty acids
◦ Some citric acid intermediates can form nucleic acids
(2/10) BR (Quiz tomorrow!)
A.
B. (Stage)
Cytoplasm
C.
D. (Stage)
E.
F.
(stage)
Mitochondrial
matrix
* Write down any specific
questions you have
regarding cellular
respiration.
1. Identify the stages or
molecules in the
diagram.
2. Which type of
respiration is shown
in the diagram,
aerobic or anaerobic?
G. (stage)
CO2 and H2O
Inner mitochondrial
membrane
A. Glucose
B. Glycolysis
Cytoplasm
C. Pyruvate
D. Pyruvate
oxidation
E. Acetyl CoA
F. Krebs
cycle
Mitochondrial
matrix
G. ETC
CO2 and H2O
Inner mitochondrial
membrane
Aerobic Respiration animation recap
Electrons
carried via
NADH
PYRUVATE
OXIDATION
3.
The evolution of photosynthesizing organisms and the
development of an O2-rich environment led to a rapid
diversification of life. Explain why there is an evolutionary
advantage to an organism that requires oxygen to live
compared to one that does not require oxygen.
4.
Muscle fatigue results from EITHER lactic acid
accumulation deactivating muscle contractions OR
muscles running out of energy reserves. Identify which
you would expect to happen during a marathon versus a
sprint and WHY.
Effect of O2 Levels on Evolution
Label the mitochondrion below. Letter “a” is pointing to the
inward fold.
a
b
c
d
e
c.
er
mbrane
d.
a.
b.
e.
(2/10) BR: Photosynthesis intro
5.
6.
7.
8.
What similarities do mitochondria and chloroplasts share?
What are the reactants and products of photosynthesis?
Why do plants perform photosynthesis?
What types of organisms rely on photosynthesis for
survival?
6.5 During Photosynthesis, Light Energy Is Converted to
Chemical Energy
Photosynthesis: energy from sunlight is stored as chemical
energy in glucose
Light
◦ 6CO2 + 6H2O
C6H12O6 + 6O2
◦ 2 pathways
I. Light reactions convert light energy into chemical
energy (ATP and NADPH)
II. Carbon-fixation reactions (Calvin cycle) use the
ATP and NADPH to produce glucose from CO2
6.5 During Photosynthesis, Light Energy Is Converted to
Chemical Energy
Light is a form of electromagnetic
radiation, which travels as a wave but
also behaves as particles (photons)
◦ When a molecule absorbs photons,
its electrons become energized or
“excited”
Generally, the excited electron will fall back down and
release energy as heat…in isolation, chlorophyll releases
energy as heat and light Fluorescence
Chloroplasts are green because chlorophyll REFLECTS
green wavelengths of light. Other wavelengths of light in
the visible spectrum are absorbed
(2/11) BR
The graph shows the absorption and action spectra of chlorophyll a, the
main pigment involved in photosynthesis. An absorption spectrum shows
which wavelengths of light are absorbed, and an action spectrum shows
the amount of photosynthetic activity at different wavelengths of light.
1.
What colors does chlorophyll a absorb?
2.
What is the relationship between light absorption and photosynthetic
activity?
6.5 During Photosynthesis, Light
Energy Is Converted to Chemical
Energy
Pigments: molecules
that absorb light
◦ Absorption
spectrum—shows
light energy absorbed
at different
wavelengths
◦ Action spectrum—
shows biological activity
of an organism at
different wavelengths
6.5 During Photosynthesis, Light
Energy Is Converted to Chemical
Energy
◦ Chlorophyll absorbs blue
and red light and reflects
green
◦ Accessory pigments (e.g., betacarotene) transfer energy to
& protect chlorophyll
◦ Pigments are arranged into
light-harvesting
complexes, or antenna
systems
◦ A photosystem consists of
antenna systems & a reaction
center
If chl a is the only pigment
that actually passes the
light energy to electrons
to start photosynthesis,
then how is it that there is
photosynthesis at 500 nm
of light?
If the chl a is
the only
pigment that
actually passes
the light energy
to electrons,
then how is it
that there is
photosynthesis
at 500 nm of
light?
• Accessory
pigments
absorb light too!
Photosystem
Based on the diagrams, what purpose
does a long hydrocarbon tail serve to
a chlorophyll molecule? What other
macromolecule have you studied that
has a hydrocarbon tail?
CHLOROPHYLL
Fatty acids in the thylakoid membrane are hydrocarbons!
The thylakoid membrane is a phospholipid bilayer just like a
cell membrane, so the hydrocarbon tail on chlorophyll anchors
it within the bilayer.
Phospholipid
Chlorophyll
Photosystem
Photosystems are embedded
CHLOROPHYLL
within integral
membrane proteins
and consist of antenna systems
surrounding a reaction center
Why do the leaves Turn Color in the Fall?
What causes the leaves to be green to begin with?
Chlorophyll a and b
The colors that we see are a reflection of the wavelengths
of light that the pigments cannot absorb.
Why do leaves turn colors in the fall?
Shorter days and cooler nights stop chlorophyll production
and drive chlorophyll out of leaves
Accessory pigments normally masked by chl are revealed
High amount of sugar in leaves leads to production of
anthocyanins (red/purple/blue)
Why do the leaves fall off of the trees, and die soon after
the color change?
Without chl, no photosynthesis, and veins carrying water &
nutrients to/from leaves are closed off
(2/12) BR: Photosynthesis
1.
2.
Write the chemical equation for photosynthesis. Then label
which reactant is oxidized and which reactant is reduced.
Consider the requirements plants need to grow & survive.
Where does most of the organic material forming the
biomass of a plant come from?
(2/12) BR
2.
Identify the inner membrane, outer membrane, thylakoid,
granum, and stroma.
a.
b.
c.
d.
e.
(2/12) Bellringer
a. granum
b. thylakoid
Copyright Pearson Prentice Hall
c. Outer mem. d. Inner mem. e. stroma
6.5 During Photosynthesis, Light Energy Is Converted to
Chemical Energy
I.
Light reactions – take place in the thylakoid membrane
◦ At photosystems chlorophyll absorbs light and releases an
excited electron
Photosystem II – electron is passed to an electron
acceptor in the ETC
Water is split to replace the electron in chlorophyll
(photolysis), producing O2
Chemiosmosis produces ATP (photophosphorylation)
Photosystem I – electron passes to NADP+ (the final
electron acceptor) which is reduced to form NADPH
6.5 During Photosynthesis, Light Energy Is Converted to
Chemical Energy
◦ Noncyclic electron transport uses 2 photosystems
Electron from PS II replaces excited electron in PS I
Electron transport chains produce ATP & NADPH
Noncyclic Electron Flow
Noncyclic Electron Transport
6.5 During Photosynthesis, Light Energy Is Converted to
Chemical Energy
◦ Cyclic electron transport uses photosystem I and
produces additional ATP for carbon-fixation reactions
Excited electron cycles back to chlorophyll
Cyclic Electron Flow
6.6 Photosynthetic Organisms Use Chemical Energy
to Convert CO2 to Carbohydrates
Calvin cycle (carbon-fixation reactions) – takes place in
the stroma
◦ 3 steps:
1. CO2 Fixation
CO2 is added to RuBP (ribulose 1,5-bisphosphate) in
a reaction catalyzed by the enzyme rubisco
Produces two 3PG (3-phosphoglycerate) molecules
2. Reduction: 3PG is reduced to form G3P
(glyceraldehyde 3-phosphate)
3. Regeneration: RuBP is regenerated
6.6 Photosynthetic Organisms Use Chemical Energy
to Convert CO2 to Carbohydrates
Rubisco catalyzes a reaction that
combines CO2 with RuBP forming 3phosphoglycerate (3PG)
RuBP is regenerated
from RuMP to
complete the cycle
3PG is reduced to
glyceraldehyde 3phosphate (G3P) in a 2step reaction
About 1/6 of G3P
molecules are used
to make sugars
6.5 During Photosynthesis, Light Energy Is Converted to
Chemical Energy
◦ Extra G3P is exported to the cytosol and converted to
hexoses (glucose and fructose)
Glucose molecules are linked together to form starch
or cellulose
The C—H bonds generated by the Calvin cycle provide
almost all the energy for life on Earth
◦ Photosynthetic organisms (autotrophs) use most of this
energy in cellular respiration to support their own growth
and reproduction
◦ Heterotrophs depend on autotrophs for chemical
energy (i.e., food) to harvest during cellular respiration
Photosynthesis Video Animation
Hydrogen bonding to other cellulose
molecules can occur at these points
The G3P
made in the
Calvin
Cycle is
involved in
the biosynthesis
of other
organic
molecules
1.
2.
3.
Explain the effect of
increasing light intensity
on the rate of
photosynthesis.
Explain the effect of
increasing temperature
on the rate of
photosynthesis.
Explain WHY the rate
of photosynthesis is
affected in these ways.
(Do the shapes of these
graphs look familiar?)
Effect of Temperature on Rate of
Photosynthesis
Rate of Photosynthesis
(2/12) Exit
Effect of Light Intensity on Rate of Photosynthesis
100
80
60
40
20
0
20
40
60
Temp (C)
80
100
(2/16) BR
1.
Get out your notes from CH 6 and write down any
concepts you are struggling to understand (oxidation,
reduction, energy coupling, substrate-level/oxidative
phosphorylation, chemiosmosis, glycolysis, pyruvate oxidation,
Krebs cycle, ETC, fermentation, pigments, light reactions,
photolysis, photophosphorylation, Calvin cycle, carbon fixation…)
Effect of Light Intensity on Rate of Photosynthesis
Rate of Photosynthesis
Effect of Temperature on Rate of
Photosynthesis
100
80
60
40
20
0
20
40
60
Temp (C)
80
100
What happens as light hits the reaction center of
a photosystem?
DPIP
An electron from chlorophyll a is sent down the
ETC, reducing NADP+ to NADPH. However, in THIS
experiment, DPIP is reduced in place of NADP+.
When DPIP is reduced as NADP+ normally would, it
will go from blue to colorless
DPIP
How will the rate of photosynthesis be measured?
When DPIP is reduced, less light is able to be
transmitted through the chloroplasts. So by measuring
the % light transmittance, the rate of photosynthesis
can be deduced.
2. HYPOTHESES: How do you expect the %
transmittance of light through the solutions to
change in each solution of chloroplasts? What would
the curves look like on each graph? Sketch them!
Remember, when DPIP is reduced it will go from blue to
colorless, and % transmittance will increase.
2. HYPOTHESES: How do you expect the %
transmittance of light through the solutions to
change in each solution of chloroplasts? What would
the curves look like on each graph? Sketch them!
Remember, when DPIP is reduced it will go from blue to
colorless, and % transmittance will increase.
Bellringer: #1. Identify a-i in the diagram.
c.
h.
a.
g.
b.
e.
f.
d.
i.
(2/10)
2.
3.
4.
5.
6.
What replaces the electron in photosystem I?
What replaces the electron in photosystem II?
What is/are the product(s) of cyclic electron flow?
What is/are the product(s) of noncyclic electron flow?
Review EXIT.
1.
2.
3.
4.
What replaces the electron in photosystem I?
Electron from photosystem II
What replaces the electron in photosystem II?
water
What is/are the product(s) of cyclic electron flow?
ATP
What is/are the product(s) of noncyclic electron flow?
ATP & NADPH




