Chemistry 30 Tasks
advertisement

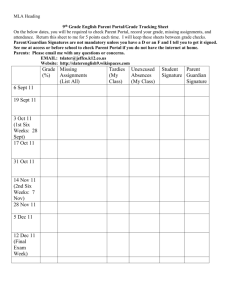
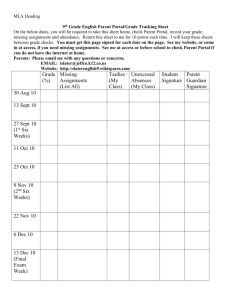
Chemistry 30 Tasks to Be Completed: Unit 1: Chemistry Review Assignment Due Date 1.Fact File Timeline and More 2. Components of an Atom 3. Calculation of Atomic Weight 4. Atomic Scientists 5.Orbital Principles 6.Filling of Orbitals 7.Electron Configuration 8.Electron Dots 9.Periodic Table and Electron Configuration 10.Chemical families and formulas 11.Chemical Families 12.Types of Chemical Bonds 13.Table of Bonding Types 14.Classification of Compounds 15.Naming Compounds 16.Calculations of Molar Mass 17.Mole Calculations using a periodic table 18.Counting Atoms 19.Balancing using inspection 20.Writing Net Ionic Equations 21.Mole Recipes 22.Mole to Mole Stoichiometry: 23.Mass to Mass Stoichiometry (Gravimetric ) 24.Gas stoichiometry 25.Review of Chemistry 20 Exam Aug 30 Aug 30 Aug 30 Sept 1 Sept 1 Sept 1 Sept 3 Sept 3 Sept 3 Sept 3 Sept 3 Sept 9 Sept 9 Sept 9 Sept 9 Sept 13 Sept 13 Sept 13 Sept 13 Sept 13 Sept 15 Sept 15 Sept 15 Sept 15 Sept 16 Unit 2: Solubility and Solutions Classification Activity Multiple intelligence Assignment on Solutions Writing dissolving reactions Molarity Calculations Assignment: Percent, ppm and ppb Dilution Calculations Standard solution and serial dilution Solubility Rules Lab Predicting precipitates and writing net ionic equations Separating Ions by Selective Precipitation Cation Analysis Calculating Ksp and Solubilities Predicting Precipitates using Ksp Solubility and Solution Exam Sept 21 Sept 21 Sept 21 Sept 23 Sept 23 Sept 23 Sept 23 Sept 24 Sept 28 Sept 28 Sept 28 Sept 30 Sept 30 Oct 1 Date Completed Mark Unit 3: Energy Changes in Chemical Reactions Energy Sources and Uses Demonstration of Molecular Enthalpy Lab Lab on Drives behind chemical reactions Graphing Relationships Heat Calculation #: using Q = m * (c) * T Assignment; Communicating Enthalpy Graphing Assignment Assignment: Equation Method Assignment: Using a Ratio Assignment: Calorimetry Calorimetry Lab Assignment: Calculation of H using Hess's Law Calculations using Hess's Law and heats of formation Graphing Assignment on Gibb's Free Energy Energy Changes Exam Oct 5 Oct 5 Oct 6 Oct 7 Oct 8 Oct 13 Oct 13 Oct 13 Oct 14 Oct 14 Oct 15 Oct 15 Oct 18 Oct 18 Oct 19 Unit 4: Rates of Reaction Graphing motion of objects. Average and Instantaneous Rates of Chemical Reactions Balanced chemical equations and rate. Factors Affecting Rate of Reaction Lab Lab on Concentration vs rate Collision theory and Surface area Collision theory and Nature of Reactants Graphical Determination of Rate Orders Method of Initial Rates Reaction Mechanisms Labs on Temperature and its Effect on Reaction Rate Catalysts and Potential Energy Diagrams Researching Catalysts Rates of Reaction Exam Oct22 Oct22 Oct22 Oct 26 Oct 26 Oct 28 Oct 28 Oct 28 Nov 1 Nov 1 Nov 3 Nov 3 Nov 4 Nov 5 Unit 5: Equilibrium Concept Attainment: Le Chatelier's Principle Assignment: Applying the generalizations Researching Applications of LeChatelier's principle Equilibrium Constant Expression Calculating Keq given equilibrium concentrations ICE Chart Questions Mole ratios and algebra ICE charts, Keq and Algebra Equilibrium Exam Nov 9 Nov 9 Nov 15 Nov 17 Nov 17 Nov 19 Nov 19 Nov 22 Nov 23 Unit 6: Acids and Bases Assignment #1: Applications of Acids and Bases Household Acids and Bases Lab Assignment #2 Bronsted - Lowry Equations Calculating [H3O+] and [OH-] Calculating [H3O+] and [OH-] of Weak acids pH calculations Understanding how a buffer works Procedure for calculating buffer ph Writing Acid / Base Neutralization Reactions Dry Lab Assignment: Plotting Changes in pH (Titration curves Titration Calculations Titration Lab Acid and Base Exam Nov 25 Nov 25 Nov 25 Nov 30 Dec 2 Dec 2 Dec 2 Dec 7 Dec. 7 Dec 10 Dec 10 Dec 13 Dec 15 Dec 16 Unit 7: Electrochemistry (Oxidation and Reduction) Writing Net and half reactions Activity: Developing Reduction Series Activity: Predicting Spontaneity Laboratory: Corrosion of Iron Diagraming Electrochemical Cells Predicting voltages of electrochemical cells Electrochemistry Exam Jan 5 Jan 5 Jan 5 Jan 7 Jan 7 Jan 11 Jan 12 Independent Study – You will pick a topic that you will research and develop a Multiple Intelligence Project for with the use of the following link: http://www.saskschools.ca/curr_content/chem30/modules/module9/multInt.htm The best thing to do is to pick a topic that you are interested in and would want to learn a little more about. Maybe you would like to learn about the use of chemistry in crime scenes, maybe you want to learn a little more about the process of painting a car, or maybe … Once you have a topic, and a plan, we will create a rubric for each group individually. This project will be due on Jan. 20.