Solubility Rules Lab - Varga
advertisement

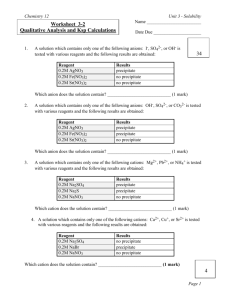
Chem 30 Solubility Rules Lab /34 Purpose: The purpose of the lab is to observe the rules of solubility and to observe the formation of a precipitate. Materials: 1. iron(II) sulfate (All salt solutions can be made by dissolving one tablespoon of solute in water and diluting to 100 ml) 2. sodium hydroxide 3. sodium chloride 4. magnesium sulfate 5. sodium phosphate 6. hydrochloric acid (dilute 500 ml concentrated HCl solution to 1.00 L with distilled or deionized water) 7. iron 8. zinc 9. distilled or deionized water 10. Test tubes 11. Pipettes Procedure: 1. Test each possible pair of solutions by combining 10 drops of each member of the pair into an empty test tube. 2. Observe to see if a precipitate forms. 3. Record your observations into the data table. 4. Repeat for ALL pairs. Follow the data table Chem 30 Data/Observations: Solubility Table: Indicate in each box whether or not there was a precipitate formed. (12 marks) Solution: NaOH MgSO4 FeSO4 CaCl2 Na3PO4 Na2CO3 NaOH MgSO4 FeSO4 CaCl2 Na3PO4 Na2CO3 NaCl For EACH reaction that formed a precipitate write out a balanced equation, indicating which ions are aqueous and solid. Next write a net ionic equation, followed by a balanced net ionic equation. (16 marks) NaCl Chem 30 Analysis: 1. Were there any reactions that formed a precipitate that was not suppose to according to the solubility chart? Any reactions that did not form precipitates that were suppose to? (2 marks) 2. What is one factor that can affect solubility? How does it affect it? (2 marks) 3. What does the term spectator ion mean? ( 1 mark) 4. Indicate at least ONE error that could occur during this lab that would alter the results. (1 mark)