Worksheet 17 3/05/14
advertisement

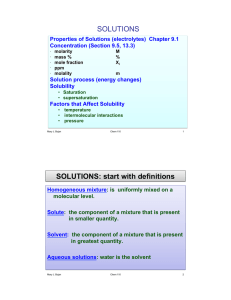
SI – Chem 178 Leader: Kyle Worksheet 17 1. How much 2M NaOH must be added to 100mL of 0.2M acetic acid in order to reach equivalence? What is the volume of the solution at equivalence? 2. 30.0mL of 0.05M NH3 is titrated with 0.025M HCl. What is the pH after the following volumes have been added? a. 0 mL b. 20.0 mL c. 59.0 mL d. 60.0 mL e. 65.0 mL SI – Chem 178 Leader: Kyle Worksheet 17 4. Write the expression for the solubility-product constant for the following: a. AgI b. SrSO4 c. Fe(OH)2 d. Hg2Br2 5. What is the difference between solubility and the solubility product constant? 6. The Ksp value for LaF3 is 2 x 10-19. What is the solubility of LaF3 in water in moles per liter?