The periodic table - Ms. Buicke maths and science
advertisement

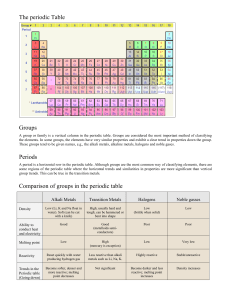
* By Ms. Buicke 1 Syllabus OC48 Describe the general properties of the alkali metals and understand that alkali metals are in Group I of the Periodic Table and have similar properties OC49 Describe the reactions of the alkali metals with air and water (word equations for reaction with water) OC50 Understand that Group II elements are the alkaline earth metals 2 What is the periodic table? All of the elements discovered to the present time are listed in a table called the periodic table of the elements. if the substance is listed there it is an element, if it is not listed there it is not an element! If you examine the periodic table you will see that each element has its own symbol. The symbols for the elements form an international language. The symbol is a short handed way to represent the element. Note: we must know the first 20 elements and 3 table! there symbols in the periodic A Russian chemist named Mendeleev arranged the elements in order of increasing mass of the atoms of each element. He also lined up elements that behaved similarly. Only about half of the elements had been discovered by this time. Mendeleev left gaps in his table for elements that had yet to be discovered Portrait of Mendeleev 4 The modern periodic table has no gaps. The elements are not arranged according to atomic mass but rather by atomic number. Elements which have similar chemical properties Are placed in columns called groups. The periodic table is an arrangement of elements in order of increasing atomic number. Groups: vertical columns of elements Periods: horizontal rows of elements in the periodic table 5 Groups: In the periodic table elements with similar properties are arranged under one another in vertical columns called groups. There are eight main groups. All elements in the same group have the same number of electrons in their outer orbit or shell. It is these electrons which determine the chemical properties of the elements. Periods: The horizontal rows of elements are called periods there are seven periods in the periodic 6 7 Group I alkali metals Potassium K Lithium Li Sodium Na Alkali metals 8 Group I alkali metals: have one electron in their outer shell. The atoms are keen to get rid of this loose electron. As a result they are very REACTIVE and behave similarly. Elements in this group include: Lithium – Li Sodium – Na Potassium – K Alkali metals are not found freely in nature. They are not found lying in the earth like gold They are so reactive they quickly form compounds Compound: two or more elements chemically combined. 9 The alkali metals must always be stored under oil this prevents them from reacting with Moisture in the air. Physical properties of the alkali metals • Soft and easily cut • Low melting points • Low density ( float in water) • Shiny when cut but quickly loose their shine 10 Alkali earth metals group 1 Potassium metal Lithium metal 11 Sodium metal Chemical properties of alkali metals. There are two chemical properties of alkali metals which we must know. Reactions of alkali metals with air: All of the alkali metals loose their shiny appearance when exposed to air. We say that they tarnish in air. The alkali metals react with the oxygen in the air forming a metal oxide Lithium + oxygen = lithium oxide Sodium + oxygen = sodium oxide Potassium + oxygen = potassium oxide 12 Reactions of alkali metals with water: When a small piece of sodium is added to water the metal floats on the surface of the water and melts into a ball, it fizzes around and burns, sparks, flames and my even explode. Hydrogen gas is released and sodium hydroxide is formed which dissolves in the water. Sodium + water -------> sodium hydroxide + hydrogen 13 When potassium is added to water a similar reaction occurs. Potassium + water potassium hydroxide + hydrogen. The least reactive of the alkali metals is lithium this reacts slowly to form lithium hydroxide and hydrogen Lithium + water lithium hydroxide + hydrogen The alkali metals react vigorously with water. Hydrogen gas is given off and a hydroxide compound is formed. 14 Reactivity increases as we go down the group of metals in group 1. Therefore Potassium is more reactive then sodium, sodium is more reactive then lithium. Why is this the case? discuss this with your partner. 15 Uses of alkali metals: Sodium is used in street lights. Lithium is used in batteries. 16 Alkali earth metals group II Beryllium – Be Magnesium – Mg Calcium – Ca All elements in this group have two electrons in their outer shell. Therefore they need to lose 2 electrons. Group II elements are not as reactive as group I elements. The alkaline earth metals don’t have to be stored under oil, as they are not as reactive with oxygen or water. 17 Calcium 2,8,8,2 Beryllium 2,2 Group II alkali earth metals. Calcium Ca Beryllium Be Magnesium Mg Alkali earth metals 18 The alkaline earth metals group II Beryllium metal Calcium metal 19 Magnesium metal Exam questions: 2012 HL Describe the reaction of a named alkali metal with water and name a product of the reaction. 2012 OL Calcium is a member of the Group II elements in the Periodic Table. (i) What name is given to the Group II elements? ______________________________ metals 2011 HL The diagram is an outline periodic table. One area, a group of elements, is shaded. Name this group of elements and give one chemical property that they have in common. Group _________________________ 20 Property_________________________ 21 http://www.youtube.com/watch?v=uixxJtJPVXk 22 23 24