Practice Chem Retake Answers Example questions to attempt after
advertisement

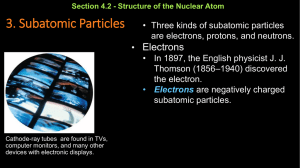
Practice Chem Retake Answers Example questions to attempt after you have studied above. 1.Look at Aluminum on the periodic table. a. How many electrons, protons, and neutrons does it have? How do you know this for each particle? 13 protons, 13 electrons, 14 neutrons b. If you added 2 electrons to it – what would you call it and why? An ion, because it would be more negative. c. If you added 2 neutrons to it – what would it be and why? An isotope, because it has a greater mass. 2. A student mixes two compounds and gets the following chemical reaction (5pts) 3H2 + 2O2 2H2O2 a. Is this reaction balanced? No – more hydrogen as reactants than products. b. What are the products? 2H2O2 c. What would be the approximate pH when the reaction is completed? No hydrogen or hydroxide, so probably neutral. 3. Draw the Bohr model and lewis dot structure for Phosphorus–for the Bohr model include the protons, neutrons and electrons. (13 pts) Chemistry Placement Retake Study Guide 1. Be able to determine the number of subatomic particles (protons, neutrons, or electrons) for any element using the periodic table. 2. Be able to determine if a substance is an element, ion or isotope from the number of subatomic particles. 3. Be able to determine if a chemical equation is balanced. Identify the products and reactants of a reaction. 4. Determine the pH of a solution (acid, base or neutral) based on the number of [H+] and [OH-] ions. 5. Draw a Bohr’s model of an atom with all three subatomic particles correctly placed and quantified. Include the number of electron cloud layers and number of electrons in each layer. 6. Draw the Lewis dot structure for any element.