Atoms & Periodic Table Worksheet: Integrated Science I
advertisement

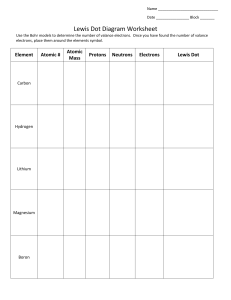
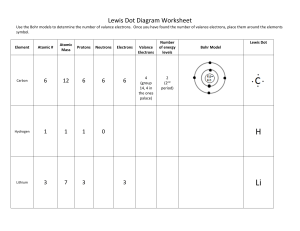
Ch. 4: Atoms and the Periodic Table – End of Chapter Integrated Science I Name: ___________________________ Date: _______________ Block: ____ 1. Complete the following crossword puzzle. Word Bank (not all will be used) Proton Neutrons Quark JJThompson Bohr Group Period Rutherford Electron Nucleus Mendeleev Metals Nonmetal Isotope 2. Complete: 1) A Bohr structure and 2) a Lewis Structure for nitrogen and neon below. Label each structure as Bohr or Lewis. 3. Complete the chart for each of the following elements Element Symbol Mass # Atomic # Protons Neutrons Electrons Valance Electrons Potassium Sulfur Boron Chlorine 4. Compare the charges of protons, electrons, and neutrons and state where each particle is located. Particle Charge Location Protons Neutrons Electrons 5. Why are noble gases stable? _______________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 6. Fill in the boxes to identify properties of the periodic table below. c. Group 17: a. Group 1: b. Group 2: e. Horizontal row: f. Vertical Column: d. Group 18: