Worksheet 7a answers - Iowa State University
advertisement
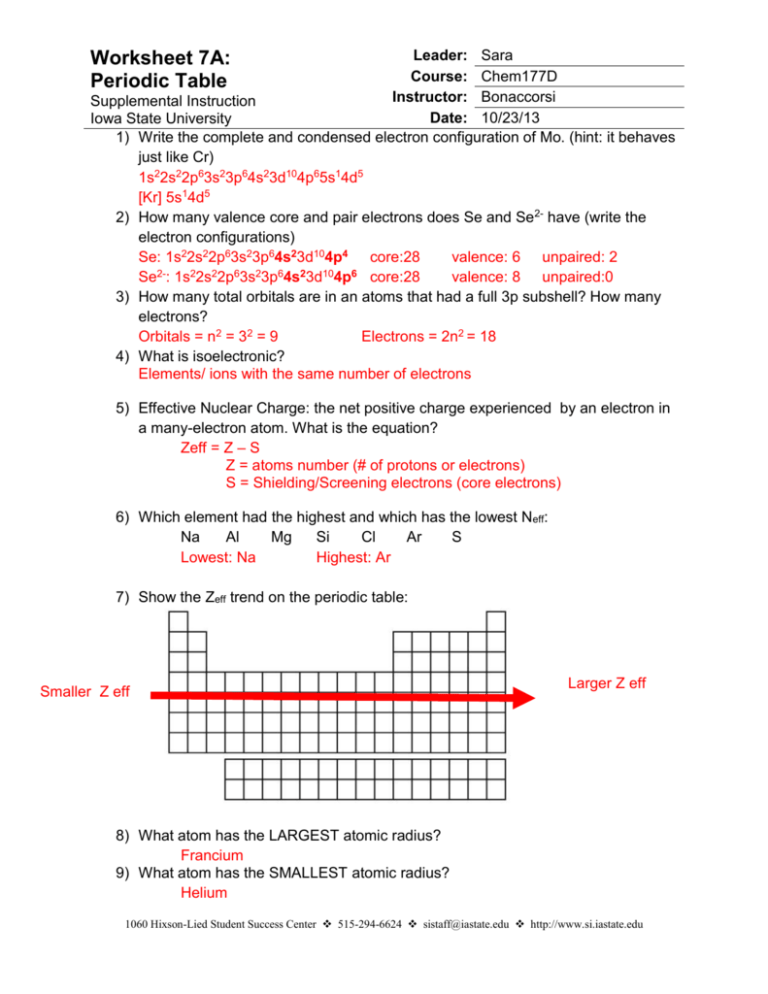
Leader: Sara Course: Chem177D Instructor: Bonaccorsi Supplemental Instruction Date: 10/23/13 Iowa State University 1) Write the complete and condensed electron configuration of Mo. (hint: it behaves just like Cr) 1s22s22p63s23p64s23d104p65s14d5 [Kr] 5s14d5 2) How many valence core and pair electrons does Se and Se2- have (write the electron configurations) Se: 1s22s22p63s23p64s23d104p4 core:28 valence: 6 unpaired: 2 22 2 6 2 6 2 10 6 Se : 1s 2s 2p 3s 3p 4s 3d 4p core:28 valence: 8 unpaired:0 3) How many total orbitals are in an atoms that had a full 3p subshell? How many electrons? Orbitals = n2 = 32 = 9 Electrons = 2n2 = 18 4) What is isoelectronic? Elements/ ions with the same number of electrons Worksheet 7A: Periodic Table 5) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons (core electrons) 6) Which element had the highest and which has the lowest Neff: Na Al Mg Si Cl Ar S Lowest: Na Highest: Ar 7) Show the Zeff trend on the periodic table: Smaller Z eff Larger Z eff 8) What atom has the LARGEST atomic radius? Francium 9) What atom has the SMALLEST atomic radius? Helium 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu 10) Which of the following molecules would you expect to be Largest? a. Br2 Cl2 Br2 b. NaCl KCl KBr NaBr KBr 11) Show the atomic radius trend on the periodic table: Smallest radius Largest radius 12) If the charge of an atom changes, does the radius of the atom change? Yes Cations are smaller, anions are larger 13) Rank the following in order of size smallest to largest: a. Ra Ca P Ga Fr O O P Ga Ca Ra Fr 14) Which is larger? a. Mg or Mg2+ b. I or I- c. K or K+ d. Se or Se2- e. Cl- or Br- f. F- or Ne g. N3- or P3- 15) Put the following in order of increasing radius: BrSe2- Sr2+ Kr RbSe2- BrKr Rb- Sr2+