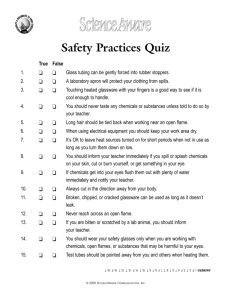
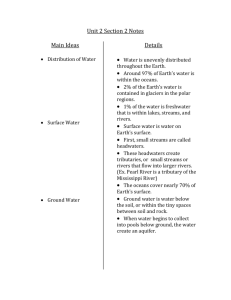
Introduction to Pollution Science
advertisement

Air, Water and Land Pollution Chapter 1: Introduction Copyright © 2009 by DBS Contents • • • • What is Pollution Science The Chemicals of Interest Units of Concentration Sources Introduction What is Pollution Science? Two key components to the definition of pollution: 1. Pollution involves a change to an environment e.g. addition of man-made chemicals that do not occur naturally* 2. The environment must be perturbed in a harmful way * This is not to say that naturally occurring chemicals are always good! They may be present at levels over and above what is healthy (criteria 2) Introduction What is Pollution Science? Not all pollutants are chemicals! What are some other examples? Here is a hint! This 1988 thermal image of the Hudson River highlights temperature changes caused by discharge of 2.5 billion gallons of water each day from the Indian Point power plant. The plant sits in the upper right of the photo — hot water in the discharge canal is visible in yellow and red, spreading and cooling across the entire width of the river. Two additional outflows from the Lovett coal-fired power plant are also clearly visible against the natural temperature of the water, in green and blue. Introduction What is Pollution Science? • Aquatic Life – Decreased ability of water to hold oxygen – Increased rate of chemical reactions – Changes in reproduction, behavior and growth throughout the food chain – Long-term damage to natural waters • Temperature is one of the most important factors governing the occurrence and behavior of life Ecological Effects of Thermal Pollution Introduction What is Pollution Science? Ecological Effects of Thermal Pollution • • • • One famous case is that of Sockeye Salmon - Columbia River A series of hydroelectric dams changed it from cool/fast-flowing to warmer/slower moving lakes Bacterial diseases drastically reduced the population Listed as US endangered species Total commercial landings of chinook and sockeye salmon in the Columbia River, 1866-1990 (from NPPC 1986, ODFW and WDF, 1991) Introduction What is Pollution Science? Introduction What is Pollution Science? Environmental Compartments Introduction What is Pollution Science? • • • Can be subdivided further May have interactions between compartments Contaminants may behave very differently in each compartment e.g. have different lifetimes (residence times), exchange rates and mobility Introduction What is Pollution Science? • When the flow of a substance into a system is equal to the outflow then the amount of substance will be constant – equilibrium or steady-state • Average amount of time a substance exists before it is removed is defined as follows: Residence time (τ) = amount of substance in the ‘reservoir’ (M) rate of inflow to, or outflow from, reservoir (F) • Must be distinguished from half-life, residence time is the time taken for the substance to fall to 1/e (~37%) of the initial concentration • Important for determining whether a substance is widely distributed in the environment c.f. CFC’s and acidic gases Question A college has a constant undergraduate enrollment of 5,000 students. No students flunk out or transfer in from other colleges and so the residence time of each student is four years. How many students graduate each year? Residence time = amount of substance in the ‘reservoir’ rate of inflow to, or outflow from, reservoir 4 yrs = 5,000 / rate of outflow Rate of outflow = graduation rate = 5,000 /4 = 1250 students / yr In this problem all students have the same residence time. In the case of pollutants residence time is an average of all the molecules and each individual molecule has different residence times Introduction What is Pollution Science? Residence time: For a 1st order reaction: C = C0e-kt When t = τ = 1/k C = C0e-1 = C = 0.37 C0 1 x C0 e (where e = 2.718..) Introduction What is Pollution Science? • Half-life: Time taken for the concentration in the reservoir to fall by 50% • When C = C0/2 C = C0e-kt C0/2 = C0e-kt e-kt = ½ τ1/2 = ln 2 k Introduction What is Pollution Science? • Monitoring: – – Chemical analysis Biological monitoring • Pros/cons: Bio-monitoring is not specific to a single substance, chemical monitoring cannot establish an adverse effect only a concentration • Two methods are complementary Introduction What is Pollution Science? • To understand the behavior of pollutants in the environment and appreciating their effects on the environment and on humans, and to monitor and manage those pollutants • A grounding in which sciences is required? Introduction The Chemicals of Interest • • • Pollution and pollutant – Preferred by environmentalists and EPA Contamination and contaminant – Preferred by US DOE Legal defination specifies concentration and location Introduction The Chemicals of Interest Inorganic Metals Metalloids Radioactive Organic Non-metals Chemical type: Heavy Metals Make connections Transition Metals Toxic Non-Toxic Introduction The Chemicals of Interest Inorganic Metals Metalloids Radioactive Organic Non-metals Chemical type Heavy Metals Transition Metals Toxic Non-Toxic Introduction The Chemicals of Interest 3 main categories: (a) Chemicals of concern because of their human toxicity (b) Chemicals which cause damage to non-human biota but are not believed to harm humans at current levels of exposure (c) Chemicals not directly toxic to humans or other biota at current environmental concentrations, but capable of causing environmental damage Introduction The Chemicals of Interest 3 main categories: (a) Chemicals of concern because of their human toxicity e.g. Pb, Cd, Hg, As have known effects No known essential role in the human body Tolerated at low-exposure Toxic symptoms Fig. 1: Introduction The Chemicals of Interest 3 main categories: (a) Chemicals of concern because of their human toxicity essential trace elements behave very differently Acceptable range Deficiency if low-exposure Toxic symptoms Introduction The Chemicals of Interest Body burdens of lead in ancient people uncontaminated by industrial lead (left); typical Americans (middle); people with overt clinical lead poisoning (right). Each dot represents 40 µg of lead. Source: Patterson et al., 1991; adapted from NRC, 1980. Case Study Minamata, 1953 • • • • • WHO limit 0.5 mg kg-1 Minamata 50 mg kg-1 • Minamata Bay, Japan (1953-1960) Plastic manufacturer (Chisso Corp.), used mercury in the production of acetaldehyde Discharged methyl mercury into the bay Main diet of locals was fish + shellfish – 5-20 ppm (106 water) Over 3,000 people suffered (730 deaths): Minamata disease / Dancing Cat Disease various deformities, damage to nervous system, retardation or death Developing embryos are especially vulnerable Health Effects Mode of Action • Hg dissolves neurons http://commons.ucalgary.ca/mercury/ Introduction The Chemicals of Interest • • Chromated copper arsenate (CCA) used to protect wood (45% As2O3) Concern over leaching of As especially in childrens playgrounds In 2004 EPA banned CCA from residential use Source: http://www.sptimes.com/News/031101/State/The_poison_in_your_ba.shtml Introduction The Chemicals of Interest • • • • e.g. Fluoride has a narrow window of optimal exposure Water is fluorinated to 1 mg L-1 Half this concentration may result in deficiency syndrome and weakened teeth Double this concentration can lead to adverse effects on teeth and bones Introduction The Chemicals of Interest • e.g. chemical carcinogens benzene, polynuclear aromatic hydrocarbons, dioxins, polychlorinated biphenyls (d) polychlorinated biphenyl (d) Introduction The Chemicals of Interest • Dioxins - Agent Orange Herbicide (defoliant) ~ 10 ppm TCDD dioxin Used by U.S. military in its Herbicidal Warfare program during Vietnam War. • Million of gallons used 1962 - 1971 to remove unwanted plant life otherwise provided cover for enemy forces during the Vietnam Conflict • Veterans and to a greater extent the Vietnamese reported a variety of health problems due to exposure that continues to this day [graphic and disturbing images] Movies: http://www.pulitzercenter.org/openitem.cfm?id=426 Introduction The Chemicals of Interest Before…… After spraying Agent Orange…… http://research.yale.edu/ysm/article.jsp?articleID=48 Baird and Caan, 2008 Introduction The Chemicals of Interest GE began using PCBs for a wide range of industrial purposes in the late 1940s. From 1947 to 1977, GE plants north of Albany poured more than 1.3 million pounds of PCBs into the upper Hudson. 200 miles is now designated NPL site By the mid-1970s, a growing number of studies had found links to premature births and developmental disorders, and had shown that PCBs caused cancer in lab animals. Today, the federal government classifies PCBs as probable human carcinogens. They are also associated with reproductive problems, low birth weight, reduced ability to fight infections and learning problems. Source: http://www.nrdc.org/water/pollution/hhudson.asp Health Effects Effects in Utero • Exposure to low levels results in impaired intellectual development NYT, September 12th 1998 Introduction The Chemicals of Interest • • • • • Polyaromatic Hydrocarbons – PAH’s Sources: oil tankers, refineries, offshore drilling, aluminum smelters, creosote (railway ties) Typically ng L-1 Larger PAH’s bioaccumulate PAH’s, PCBs and Mirex implicated in devastation of beluga Whales in the St. Lawrence River Introduction The Chemicals of Interest 3 main categories: (b) Chemicals which cause damage to non-human biota but are not believed to harm humans at current levels of exposure e.g. Copper and zinc are essential trace elements for humans Toxic to growing plants and there are regulations limiting their addition to soil in materials such as sewage sludge Introduction The Chemicals of Interest 3 main categories: (b) Chemicals which cause damage to non-human biota but are not believed to harm humans at current levels of exposure e.g. Endocrine disrupting chemicals hormone mimics which disrupt reproduction and growth in wildlife Introduction The Chemicals of Interest Baird and Caan, 2008 Introduction The Chemicals of Interest 3 main categories: (c) Chemicals not directly toxic to humans or other biota at current environmental concentrations, but capable of causing environmental damage e.g. CFCs disrupt stratospheric ozone cycles Turco, 2002 Introduction The Chemicals of Interest 3 main categories: (c) Chemicals not directly toxic to humans or other biota at current environmental concentrations, but capable of causing environmental damage e.g. Carbon Dioxide - the greenhouse effect Introduction The Chemicals of Interest Introduction The Chemicals of Interest http://services.google.com/earth/kmz/changing_sea_level_n.kmz Introduction Units of Concentration • When describing environmental processes it is important to know how much of each participating chemical is present • Confusing to the newcomer Introduction Units of Concentration Soilds – mass analyte/total mass of sample • mass per unit mass, also known as w/w e.g. mg Pb / kg soil (mg/kg, mg kg-1) • Important with soils to note wet or dry weight due to moisture content Introduction Units of Concentration Aquatic systems – mass analyte/total mass of sample • mass per unit mass, ng kg-1 or μg kg-1 Or more commonly mass analyte/total volume of sample • Mass per unit volume, mg L-1 (=ppm) or μg L-1 (=ppb) and ng L-1 (=ppt) • Unfortunately leads to confusion with units used in atmospheric chemistry which have a different meaning (volume per unit volume v/v instead of w/v) Introduction Units of Concentration ppmw vs. ppmv e.g. 10 mg F per million mg of water = 1 g F per million g of water = 10 tons F per million tons water = 10 mg F per million mg water = 10 ppmw F (or ppm/w) [since 1000,000 mg water = 1 kg water = 1 L water] = 10 mg F per L water (10 mg/L or 10 mg L-1 F) = 10 ppmv F (or ppm/v) ppm = mg kg-1 = mg L-1 Introduction Units of Concentration • ppm, ppb, etc. (assumes pollutant has same density as water, ρ = 1.00 g mL-1) e.g. show that 1 mg/L = 1 ppm 1 g pollutant 1 mg pollutant /L of H 2O 1000 mg 1 mg/L = 1 ppm 1 μg/L = 1 ppb 1 ng/L = ppt Conversions: 1 ppb = 1 ppm / 1000 1 ppt = 1 ppb /1000 1 L H 2O 1 g pollutant = 1 part per million 6 1000 g H 2O 10 g H 2O Question Prove that 1 mg L-1 = 1 μg mL-1 1 mg x 1000 μg mg L x 1000 mL L = 1 μg / mL Question Example 1: convert 0.100 M lead nitrate to ppm First convert mol/L to g/L, then to mg/L (ppm) M[Pb(NO3)2] = 331.2 g/mol 0.100 mols/L = 0.100 mols x (331.2 g/mol) / 1 L = 33.1 g / L = 33.1 g/L x 1000 mg/g = 33100 ppm Example 2: convert 0.01 g lead nitrate dissolved in 1L to ppb First convert g/L to mg/L (ppm), then ppm to ppb 0.01 g/L x (1000 mg/g) = 10 mg/L = 10 ppm 10 ppm x 1000 ppb / ppm = 10,000 ppb Introduction Units of Concentration Atmosphere – Concentration units for gases • Mass per unit volume, μg m-3 (=ppm) or molecules cm-3 • Not independent of temperature or pressure, volume of air will change, mass of pollutant won’t change e.g. air containing 1 μg m-3 SO2 at 0 °C will contain less than 1 μg m-3 SO2 if heated to 25 °C Introduction Units of Concentration Atmosphere – Mixing ratios volume analyte/total volume of sample • Problem is overcome by expressing concentration of a trace gas as a volume-mixing ratio, e.g. 1 cm3 SO2 dispersed in 1 m3 air = 1 ppmv • 1 ppmv • Now if T or P changes effects both trace gas and air in which it is contained, volume-mixing ratio does not change = 1 molecule in 106 molecules = 1 mol in 106 mols = Partial pressure of 10-6 atm. Introduction Units of Concentration: Gases • Conversion (at normal temperature of 20 ºC and 1 atm.) from w/v to v/v: concentration (ppmv) = concentration (mg m-3) x 24.0 Molar mass Note: At STP of 273 K (0 C) the molar volume is 22.4 • Similarly: concentration (ppbv) = concentration (μg m-3) x 24.0 Molar mass concentration (pptv) = concentration (ng m-3) x 24.0 Molar mass Question Convert 800 mg m-3 O3 (w/v) to ppm (v/v) without short-cut M [O3] = 48 g mol-1 No. moles O3 in 1 m3 air = 800 mg / 48 g mol-1 = 800 x 10-3 g / 48 g mol-1 = 1.67 x 10-2 mol Volume occupied by 1 mole at 20 °C and 1 atm (SATP) = 24.0 L = 0.0240 m3 Volume O3 in 1 m3 air = 1.67 x 10-2 mol x 0.0240 m3 mol-1 = 400 x 10-6 m3 = 400 ppmv Question Convert 800 mg m-3 O3 (w/v) to ppm (v/v) with short-cut concentration (ppmv) = concentration (mg m-3) x 24.0 Molar mass = 800 mg m-3 O3 x 24.0 = 800 mg m-3 O3 x 0.5 = 400 ppmv 48 g mol-1 Question Express [O3] = 2.0 x 1012 molecules cm-3 as a volume mixing ratio (ppbv) [Convert to mg m-3 then use w/v to v/v conversion] [O3] = 2 x 1012 molecules cm-3 = 3.3 x 10-12 mols cm-3 = 3.3 x 10-12 mols cm-3 x 48 g/mol = 1.6 x 10-10 g cm-3 = 1.6 x 10-7 mg cm-3 x (1 x 106 cm3 / m3) = 0.16 mg m-3 = 0.16 mg m-3 x 24.0 / 48 g mol-1 = 0.080 ppmv = 0.080 x 1000 ppmv / ppbv = 80 ppbv Question Calculate the pressure of ozone in atm and in ppmv at the tropopause (15 km, 217 K), given [O3] = 1.0 x 1012 molecules cm-3, and p(total) = 0.12 atm [O3] = 1.0 x 1012 molecules cm-3 x 1000 cm3/1 L x 1 mol/6.022 x 1023 molecules = 1.7 x 10-9 mol L-1 pV = nRT, p(O3) = (n/V) RT = 1.7 x 10-9 mol L-1 x 0.0821 L atm/mol K x 217 K = 3.0 x 10-8 atm p(O3) ppmv = (3.0 x 10-8 atm / 0.12 atm ) x 106 ppmv = 0.25 ppmv Introduction Sources • • Point – well-defined source (e.g. end of a pipe, smokestack, drain) Non-point - less well defined, cannot be pinpointed Arbitrary to some extent – depends on spatial scale e.g. air - one smoke stack (P) meaningless to analysis of regional air pollution, 100’s of stacks become NP source e.g. water - one house septic system (P), on a regional scale may be considered NP Discharge from waste water plant contaminates ground and surface water Source: USGS Point and Non-point Sources Smol, 2008 Introduction Sources Source General Waste (representative) Agricultural Field and chemical waste, nutrients, pesticides/herbicides, petroleum fuels, feedlot waste, dairy waste Chemical Industry Metal products, metal sludges, nonmetal waste, electrical equipment waste, detergents/soaps/cleaners, petroleum, metal plating, film processing, solvents, wastewaters, pesticides, smog precursors (NO X, HC’s) Mining Industry Mine tailings, Mineral leachate (CN), acid mine drainage, coal, smelting waste, particulates Energy Industry Petroleum-based waste, solvents, gas and vapor emissions, coal tars, boiler waste, nuclear waste, petroleum stored underground, smog and acid rain precursors ((NOX, HC’s) Landfills Chemicals Incinerators Incomplete combustion of feedstock, combustion by-prodcuts, metals, particulates Medical Industry Biohazards, pharmaceutical waste, solvents Food Processing Waste food products, rinsing waste, slaughterhouse waste Domestic Waste Detergents/cleaners, pesticides, fertilizers, compost, paints/solvents, gasoline Municipal Governments Water and wastewater treatment chemicals, sewage Federal Givernemnt Weapons-related waste, nuclear waste, petroleum-based waste Dunnivant and Anders, 2006 Introduction Sources • Basel Convention: International treaty regulating reporting, disposal and transport of hazardous waste • Designed to reduce movement of waste • Nuclear waste not included! (Data from 2000, metric tons) http://www.basel.int/natreporting/index.html Questions 1. Correlate hazardous waste to a country’s development level (economic status). 2. Calculate the import/export ratio (if ratio > 1 the country is a net importer). Are there any net importers? 3. Why are Germany and Japan absent from the list? 4. How does the US hazardous waste amount compare to the rest of the world? 5. Why is the US (40,821,482 tons in 2001) absent? Questions 1. Which state is the largest producer of hazardous waste? 2. Which has the most hazardous waste generators? 3. Conduct an internet search of a company from any state and determine what are their waste chemicals. Data Large quantity generator http://www.epa.gov/epaoswer/hazwaste/data/biennialreport/ Data Introduction Summary • • • • Pollution is the introduction of contaminants (chemicals, heat, light, noise) into an environment that causes instability, disorder, harm or discomfort to the physical systems or living organisms they are in Chemicals Units Sources References • • • Harrison, R.M. (2006) Introduction to Pollution Science. The Royal Society of Chemistry, London. Dunnivant, F.M. and Anders, E. (2006) A Basic Introduction to Pollutant Fate and Transport: An Integrated Approach with Chemistry, Modeling, Risk Assessment, and Environmental Legislation. Wiley-Interscience, New Jersey. Smol, J.P. (2008) Pollution of Lakes and Rivers: A Paleoenvironmental Perspective. Wiley-Blackwell. Introduction Harrison • This chapter is available as a publisher’s Adobe PDF file here: http://academics.rmu.edu/faculty/short/envs4450/textbooks/Harrison-IPS-Chp1.pdf